- Home
Welcome !
Welcome to john houghton's home page for his biology courses. This site is designed as a hub for curating and sharing lectures, course syllabi, assignments, and links to relevant resources. Use the menu bar at the top of the screen to navigate through the site.
(Please note: this page is currently under construction.)

- BIOL 2107
Fall '22 CRN87989
Lectures: (1)
- Courses
- Resources
General Resources

Life of the Cell -ANIMATION Inner_Cell_Transport -ANIMATION
Term Paper:
1. "Evolution is a man-made myth"2. "An understanding of Genetics is fundamental to our understanding of how an organism works."
3. "SARS CoV-2 pandemic was / is overblown" -a Scientific assessment
Choose one of the statements above, and provide two arguments for me; one for and one opposed to the statement that you chose.
Minimally, each of your arguments should be half a page of 11pt, single-spaced writing (450 words).
Maximally, each of your arguments should be no more than one page of 11pt, single-spaced writing (900 words).
In addition: you will need to put down references for all the sources of information that you cite.
You will submit your paper as a typed document (E-MAIL)... by NOV 18th!!
When you do e-mail me your paper, please ensure that you give the title "BIO2107 Term Paper" in the subject line of the email.
Example: Term Paper
___________________
Quantitative analysis measures the amounts of substances and solvents. Chemists are really finicky about quantification...we biologists should be more so.
Importance of Molecular weights (mass), Moles (molar) = (MW in g / per litre) pH etc.. hopefully covered in the review (last lecture).
As we have previously discussed -you need to be able to understand how mass and volume are measured relative to cells and size of cells...just as geologists and astrophysicist relate to geological age,
Macromolecules: Giant
Polymers
There are four major types of biological macromolecules: proteins, carbohydrates, lipids (?), and nucleic acids.
These macromolecules are made the same way in all living things, and they are present in all organisms in roughly the same proportions.
Macromolecules are essentially giant polymers, which are formed by covalent linkages of smaller units called monomers.
Molecules with molecular weights greater than 1,000 Daltons (atomic mass units) are usually classified as "macromolecules".
Some of the many roles of macromolecules include:
Energy source
Energy storage
Structural support
Catalysis
Transport
Protection and defense
Regulation of metabolic activities
Maintenance of homeostasis
Means for movement, growth, and development
Heredity
The
diverse functions of macromolecules are
invariably related to their shape and the
chemical properties of their monomers.
Condensation Reactions
Macromolecules are made from smaller monomers by the removal of water. This is called a condensation (loss of water) reaction.
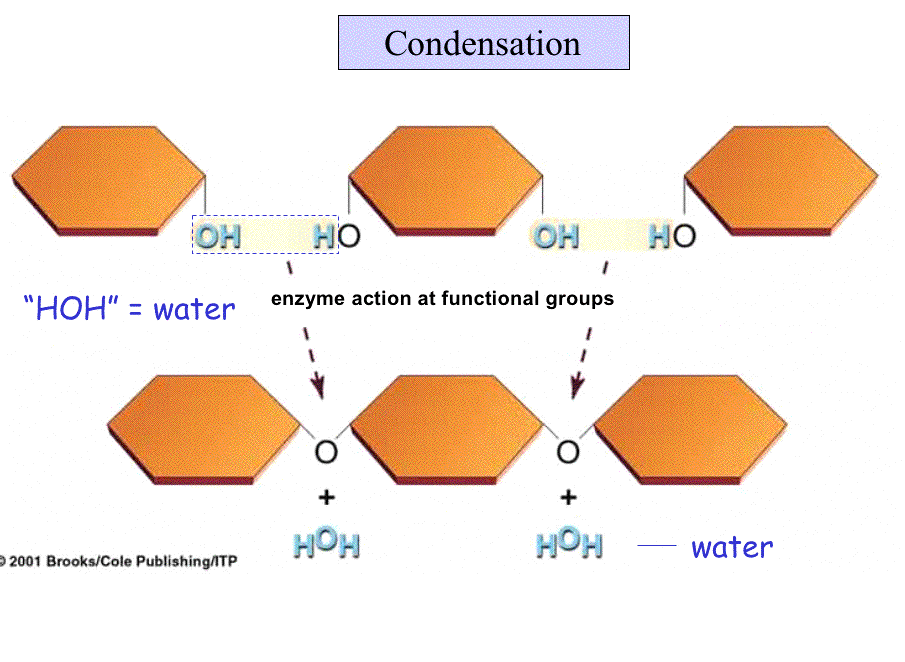
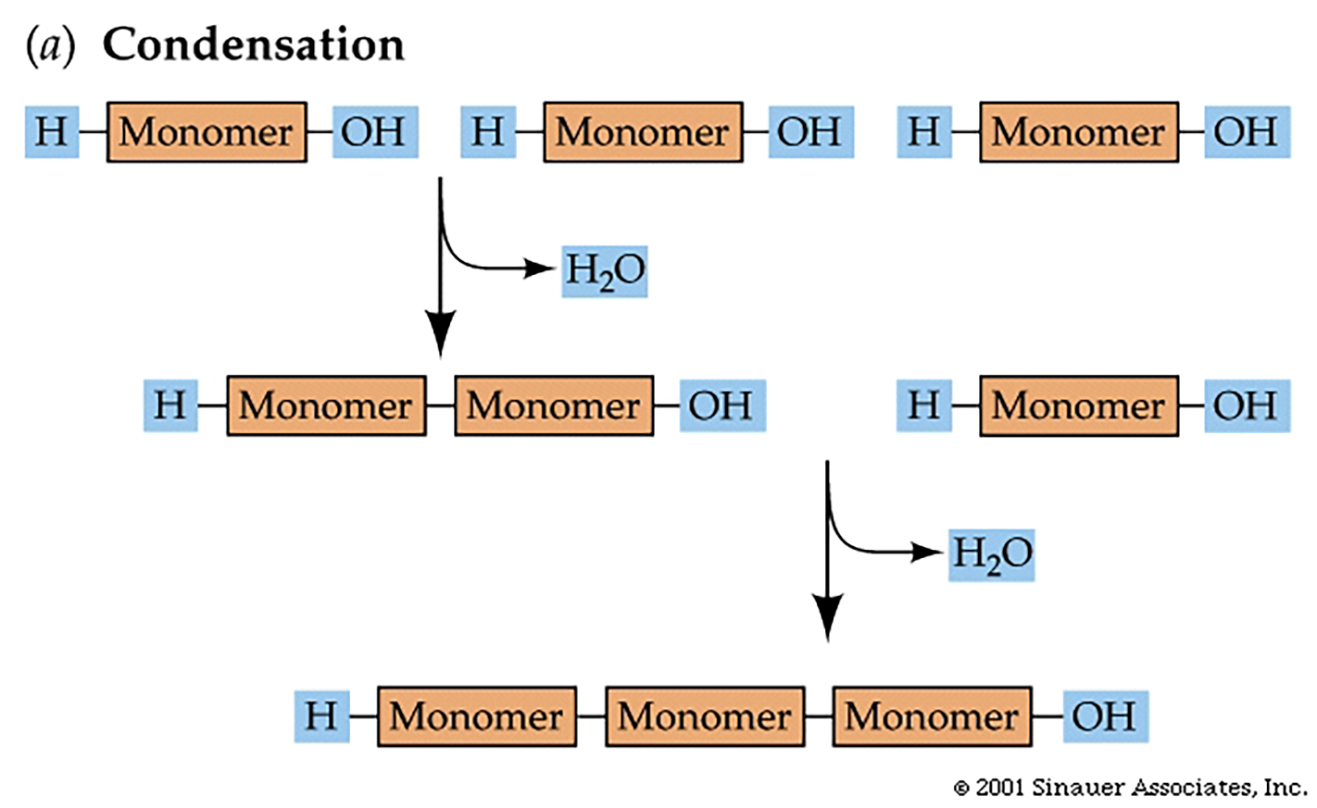
Energy must be "added" to make or break a polymer.
The reverse reaction, breaking polymers back into monomers, is a hydrolysis, which involves the addition of water; reacting with the bond that links the units together.
Proteins: Polymers of Amino Acids

Proteins
are molecules with diverse structure and
function.
Proteins have important roles including:
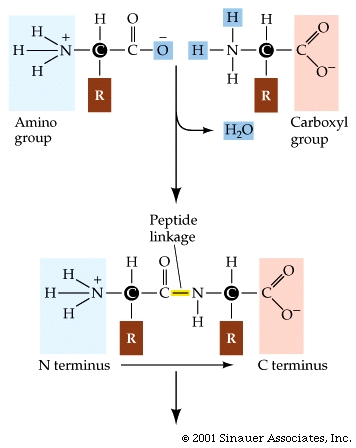
Proteins are synthesized by condensation reactions between the amino group of one amino acid and the carboxyl group of another. This forms a peptide linkage.
Enzymes increase the rates of chemical reactions in cells. This function is known as catalysis.These enzymes are highly specific; in general, each enzyme catalyzes only one chemical reaction.
Proteins range in size from a
few amino acids to one or more thousand.
Some proteins are composed of a single chain of amino acids, called a polypeptide.
Other proteins, as we have seen, have more than one polypeptide chain.
Folding of the polypeptide chain is crucial to the function of most protein, a factor that is largely influenced by the sequence of its componment amino acids.
Each different type of protein has a characteristic amino acid composition and order.
Differences in amino acids come
from the side chains, or the R groups, found attached to
the same carbon as the amino group.
The 20
common amino acids vary widely in properties. All but one have
four different groups that are attached to the alpha-carbon.
Hydrogen atom, an amino group, and a carboxyl group are bonded to the alpha-carbon
of all the different amino acids.
The first amino acid of a peptide is called the N-terminus amino acid because the amino group is free, or unbound. The last is called the C-terminus amino acid and has a free carboxyl group.
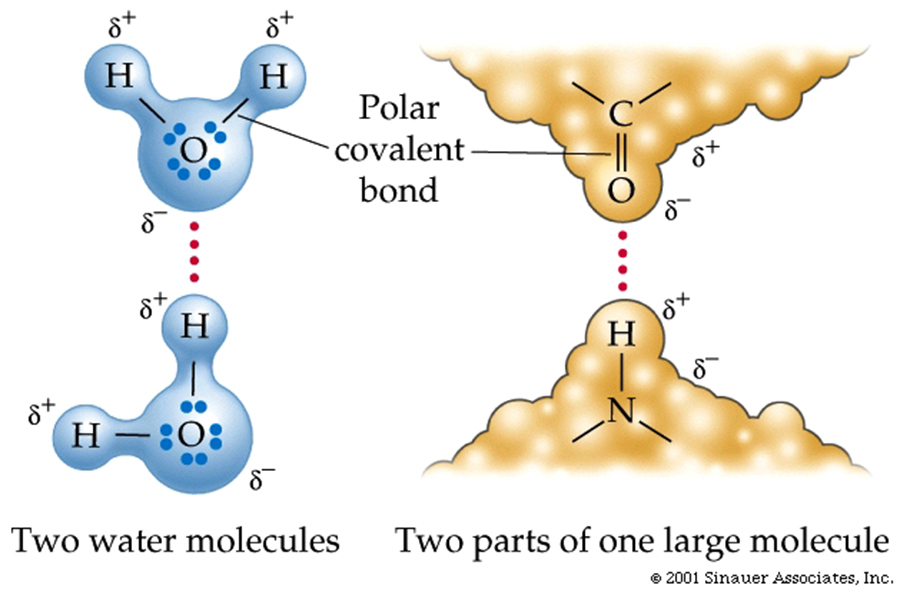
The C–N peptide linkage forms a partial double bond, which is a single covalent and polar attraction. This bond limits folding and restricts the ability of the adjacent atoms to rotate.
Within the central axis of the protein, there is an asymmetry of charge favoring a tendency toward hydrogen bonding. (Oxygen is partially negative and nitrogen is slightly positive.)
The primary structure of the protein is its amino acid sequence.
In the three-letter system, methionine is Met; in the one-letter system, it is M, but while Ala is A, Asp is D. You will (eventually) need to learn them.
Given the 20 potential building blocks there are potenially an enormous
number of different proteins, although not all variations are biologically
functional.
Secondary structure is the shape regions of the peptide take on as a folded polymer.
This shape is influenced primarily by the amino acid sequence (the primary
structure).
There are two common secondary structures: one
is the alpha-helix,
which is a right-handed coil.
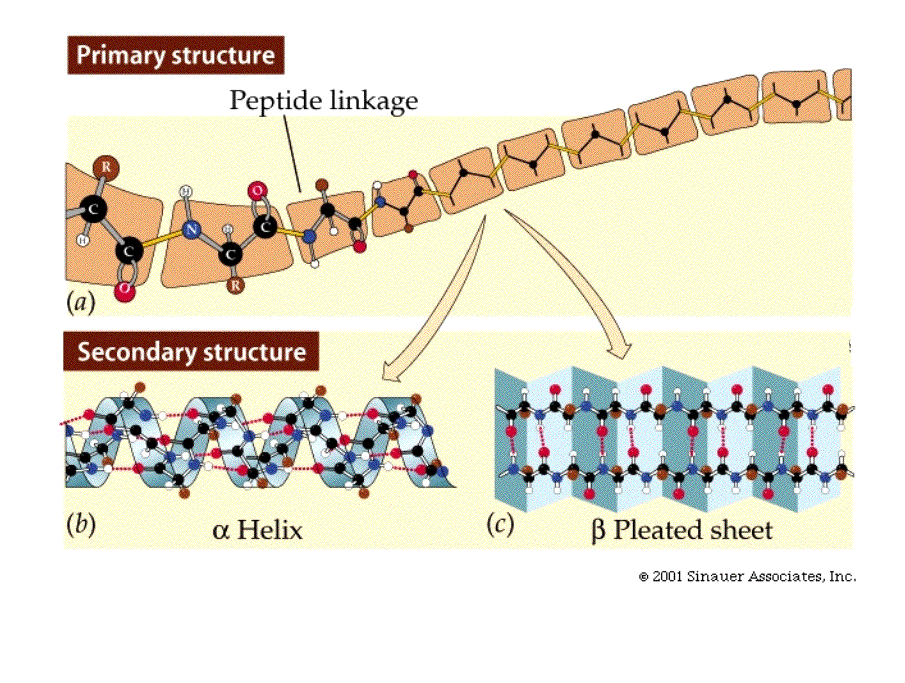
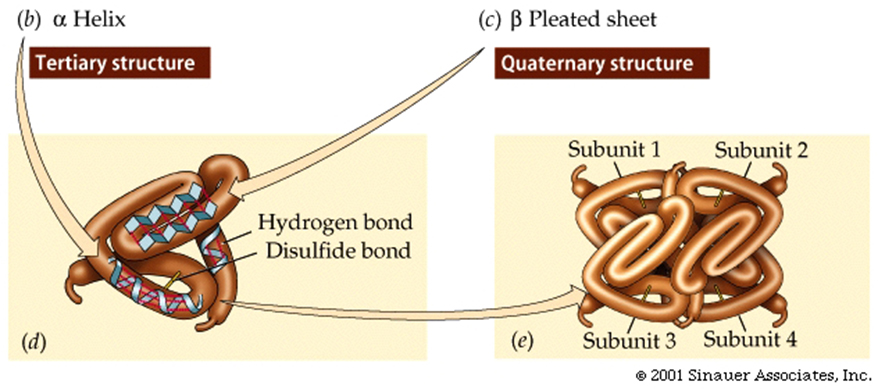
Note that in this structure,
the peptide backbone takes on the helical shape due to hydrogen bonds with
its "R groups" pointing away from the peptide backbone.
Insoluble fibrous structural proteins tend
to have a-helical
secondary structures.
There are other structures....but
not for this course.
Tertiary structure is the three-dimensional shape of the
completed polypeptide.
The quaternary structure of a protein consists of subunits
Some proteins are composed of subunits, which are separate peptide chains
that associate together to create the functional protein.
Quaternary structure results
from the ways in which multiple polypeptide subunits bind together and interact.
Hemoglobin is an example of such a protein; it has four subunits.
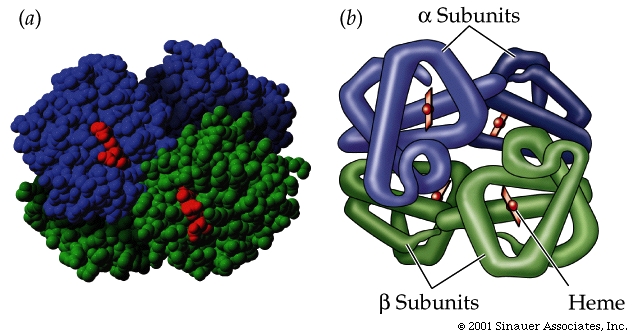
Carbohydrates:
Sugars and Sugar Polymers
Carbohydrates are carbon molecules with hydrogen and hydroxyl groups.
They act as energy storage and transport molecules.
They also serve as structural components.
Carbohydrate
monomers have molecular weights of approximately 100 Daltons.
Polymers composed of monomers can have molecular
weights of up to hundreds of thousands of
Daltons.
There are four major categories of carbohydrates:
Monosaccharides, Disaccharides,
which consist of 2 x monosaccharides and Oligosaccharides,
which consist of between 3 and 20
monosaccharides.
Finally there are Polysaccharides, which are composed of hundreds to thousands of monosaccharides.
The general formula for a carbohydrate monomer is multiples of CH2O, maintaining a ratio of 1 carbon to every 2 hydrogens and 1 oxygen.
During the polymerization process, which essentially is a series of condensation reactions, the carbohydrate polymers have ratios of carbon, hydrogen, and oxygen that differ somewhat from the 1:2:1
Monosaccharides are simple, single sugars, such as glucose (C6H12O6).
Green plants and some bacteria produce monosaccharides directly; other organisms acquire glucose and/or metabolize other sugars into this energetically favourable molecul, or an equivalent.
Cells break down glucose to release
energy, with the final products being carbon dioxide and water.

The ring form is the predominant form in the cell (>99%). There are two forms
of the ring: a-glucose
and b-glucose.
The two forms exist in equilibrium when
dissolved in water.
Different monosaccharides have either different numbers or arrangements of carbons. with most monosaccharides having what are called optical isomers.
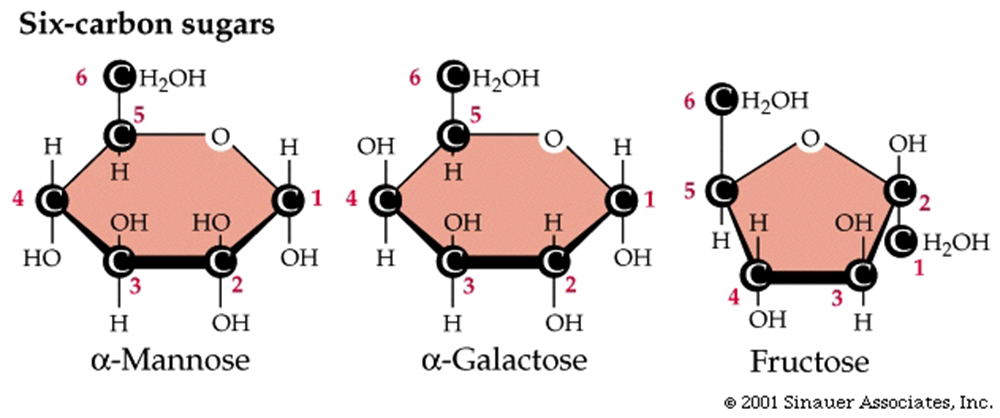
Hexoses (six-carbon sugars) include
the following structural isomers: glucose, fructose, mannose, and galactose.
Two examples of pentoses (five-carbon sugars) are ribose and deoxyribose,
which make up the backbones of nucleic acids (RNA
and DNA).
Deoxyribose is
missing an oxygen atom at carbon 2'. This results in a functional distinction
between DNA and RNA that plays a significant role in what each can and cannot do.
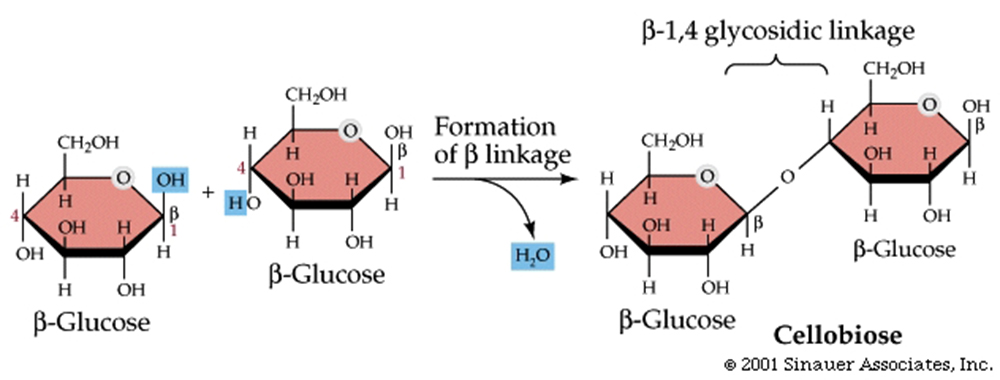
Glycosidic linkages bond monosaccharides together
Glycosidic linkages are created by enzymes and are condensation reactions.
Disaccharides have just one such linkage.
Sucrose (table sugar) is
glucose bonded to a fructose.
Lactose (milk sugar) is glucose bonded to a galactose.
Maltose has two a-linked glucose molecules.
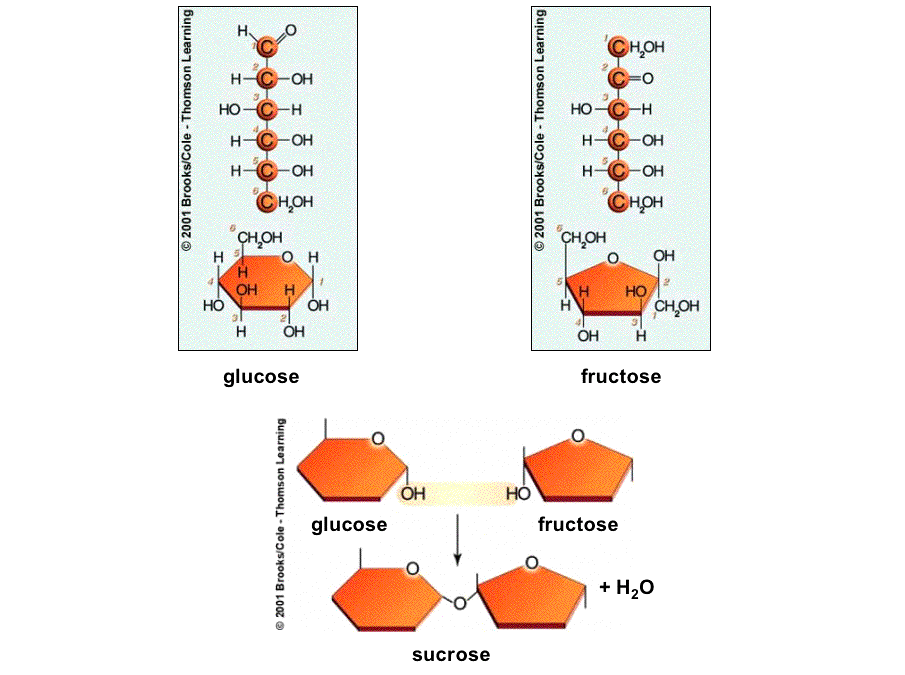
Oligosaccharides contain more than two monosaccharides.
Many proteins found on the outer surface of cells have oligosaccharides
attached to the R group of certain amino acids, or to lipids.
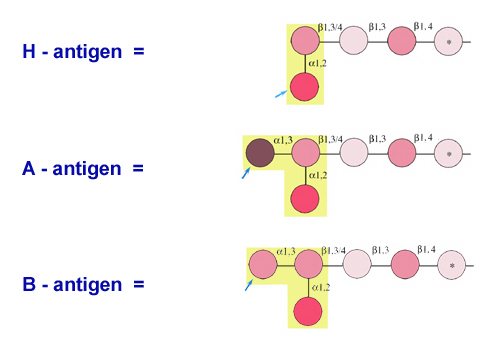
Remember the human ABO blood types antigens, for example, owe their specific oligosaccharide
chains that are attached to he red blood cells.
Polysaccharides serve as energy stores or
structural materials.
Polysaccharides are giant chains of monosaccharides connected by glycosidic
linkages.
Cellulose is a giant polymer of glucose alone joined by ß-1,4 linkages.
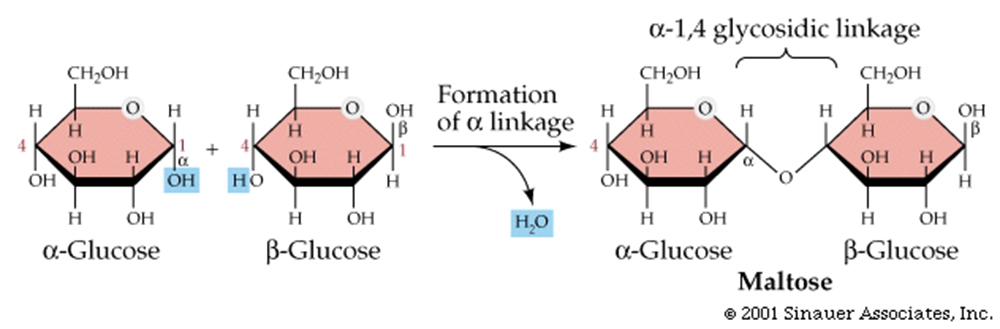

Starch is a polysaccharide of
glucose with a-1,4 linkages.
Starch can be readily degraded by the action of chemicals or enzymes, making
it a good storage medium.
Cellulose is much more
stable chemically than starch and more difficult to hydrolyze chemically
and enzymatically. This quality makes it an excellent structural material.
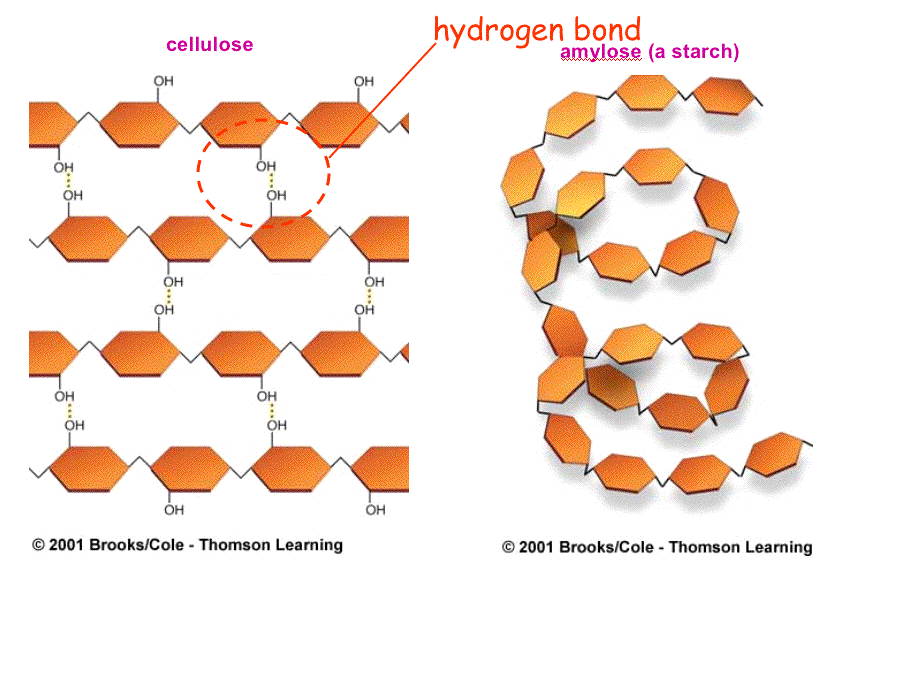
Starches vary by amount of branching.
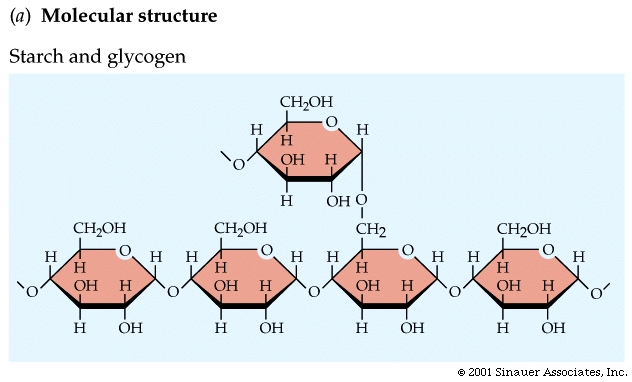
Plant starch, called amylose, is slightly branched.
Animal starch, called glycogen, is highly branched.
Starches are molecules that store glucose.
Each polymer molecule has essentially the same effect as one monomer molecule on the osmotic pressure of a solution.
The addition of functional groups modifies carbohydrates.
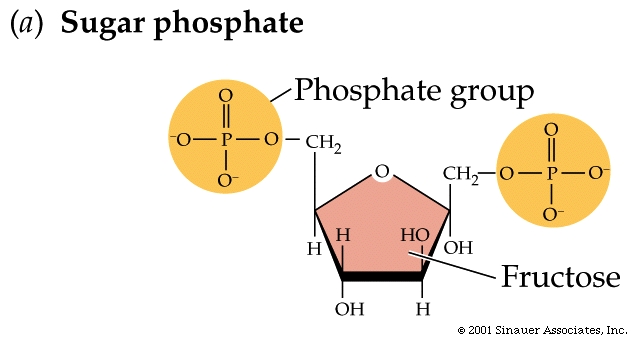
Glucose can oxidize to acquire
a carboxyl group (—COOH), producing glucuronic acid.
Phosphate is enzymatically added to one or more of the hydroxyl (—OH)
sites, creating a sugar phosphate such as fructose 1,6-bisphosphate.
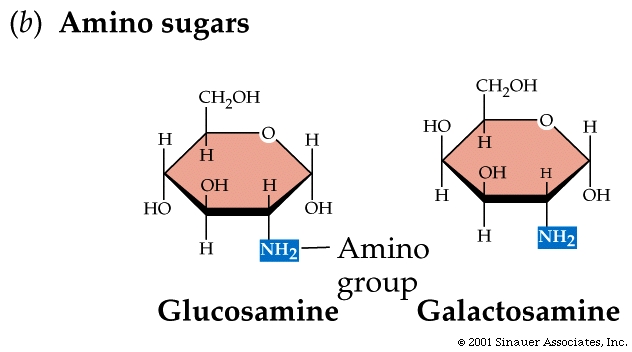
Amino sugars are important to
the extracellular matrix, the systems that hold tissues together.
Galactosamine is a major component of cartilage, which is found in your
ears, nose, and kneecaps.
A glucosamine derivative is a component of chitin, the polysaccharide in
the skeletons of insects, prawns, and crabs. It is also found in the cell
walls of fungi. Chitin is
one of the most abundant substances on earth.
Nucleic Acids: Informational macromolecules.
Nucleic acid polymers are linearly arranged, stucturally repetative sequences that function more from there sequence within the assembly more than from any chages in structure of the molecule.
Two types of nucleic acid polymers are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
Purines have a fused double-ring
structure.
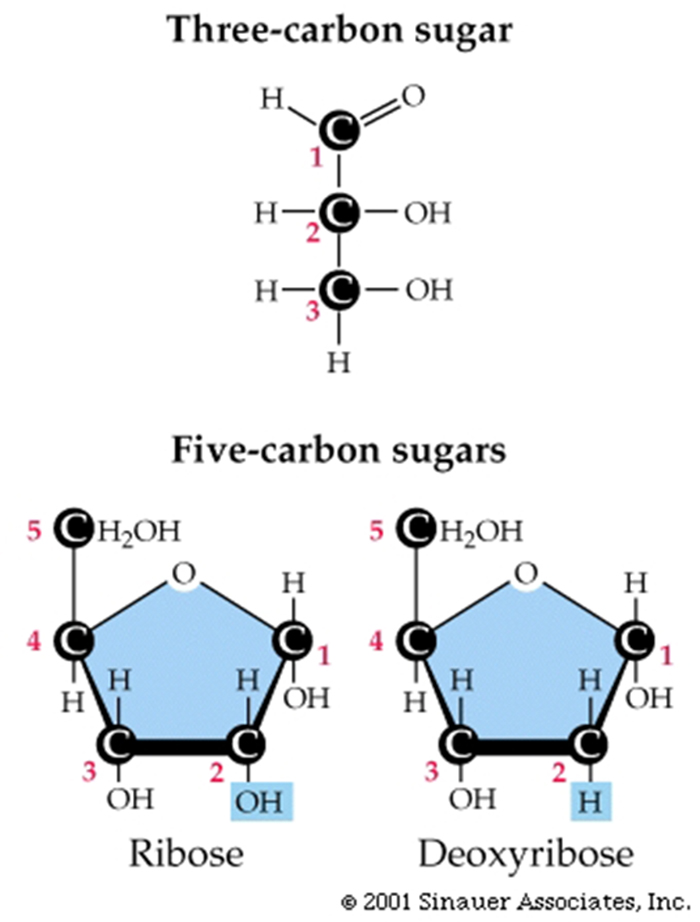
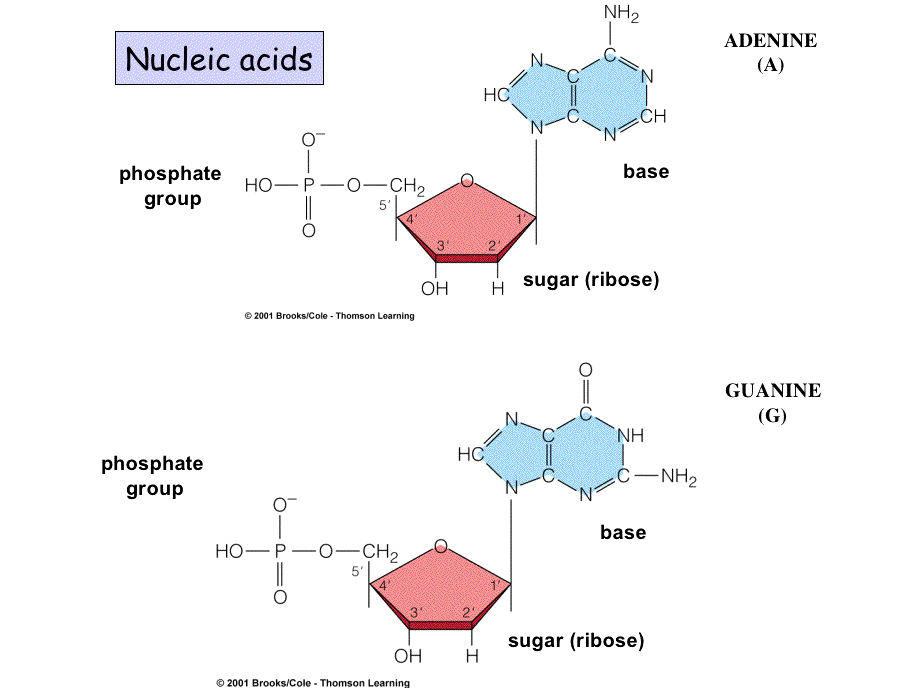
Pyrimidines have just one ring.
Pairing of a purine with a pyrimidine maintains three rings in the center
of the molecule, so the backbones of the two strands maintain a constant distance along the length of the
double-stranded molecule.

DNA and RNA polymers are enzymatically
made and, like all the other polymers mentioned so far, are created with
condensation reactions.
The linkages that hold the nucleotides in the polymer are called phosphodiester
linkages.
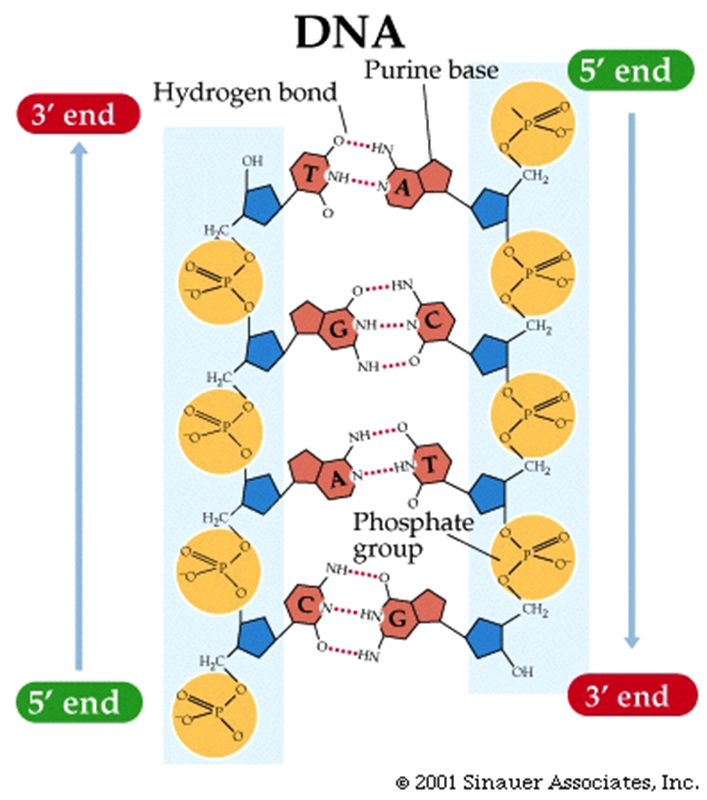
These linkages are formed between carbon 3 (the 3' carbon) of the sugar (ribose in RNA, deoxyribose in DNA) and a phosphate group that is associated with the carbon 5 (the 5' carbon) of the sugar.
The backbone consists of alternating sugars and phosphates.
In DNA, the two strands are antiparallel: Looking at one end, one strand ends with a free 5' carbon of the deoxyribose, the other with a 3' of the deoxyribose.
The two strands are held together by the attractions (hydrogen bonds) formed by nitrogenous bases in the centre of the double-stranded molecule.
The attractions are hydrogen bonds that form due to partial positive and negative charges, as described in the previous lectures.
So, is that it for chemistry....well not quite, because while we have discussed protein structure, information structues and potential food and storage material, what about the very structure that holds all these components together...lipids.
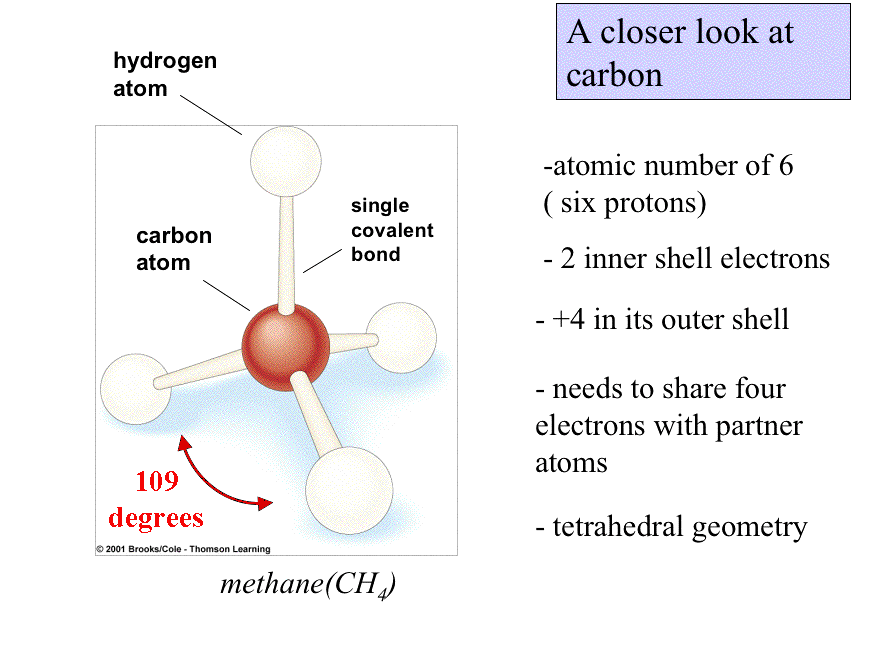
Lipids are in essence the "water-insoluble" molecules of life.
They are extremely diverse biological molecules that share a series of common chemical properties:
Their inherent insolubility results from the numerous nonpolar covalent bonds beteween hydrogen and carbon in lipids.
Lipids aggregate AWAY from water, which is polar, and are attracted to each other via weak, but additive, hydrophobic and van der Waals forces.
Lipids are not "technically" polymers, because the different units and subunits are not necessarily held together by covalent bonds, but by other less well defined forces of association.
The roles for lipids in organisms include
energy storage (fats and oils),
cell membranes (phospholipids),capture of light energy (carotinoids),hormones and vitamins (steroids and modified fatty acids),thermal insulation,electrical insulation of nerves etc,and
water repellency (waxes and oils).
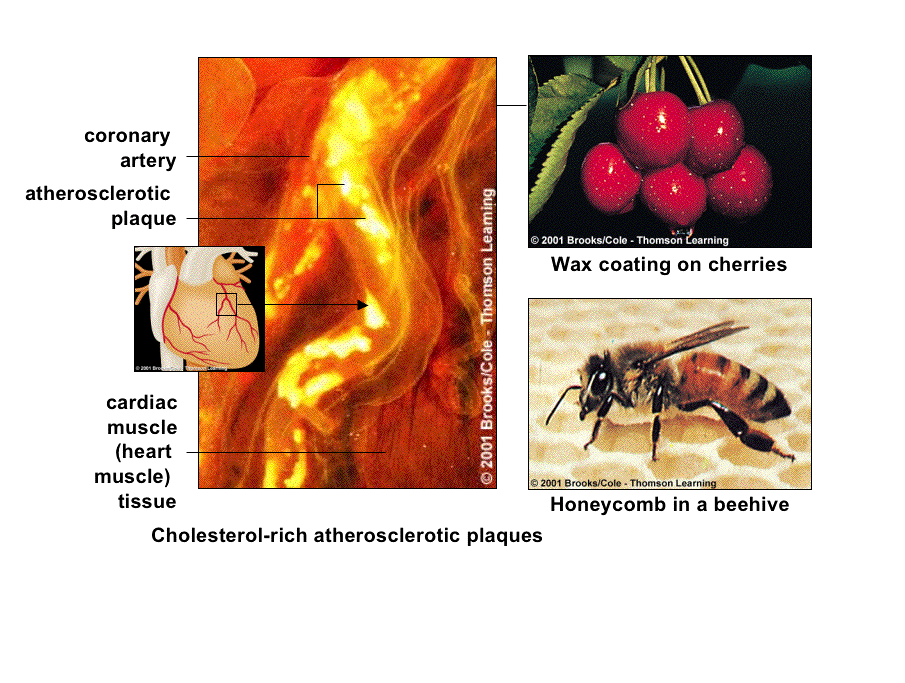
Fats and oils are triglycerides, or "simple" lipids composed of three fatty acid molecules and one glycerol molecule.

Glycerol (or glycerin) is a three-carbon molecule with three hydroxyl (—OH) groups, one for each carbon.
Each —OH is the site where an enzyme adds a fatty acid.
What is lost when it condenses?Fatty acids are long linear chains of hydrocarbons terminating with a carboxyl group (—COOH).
In saturated fatty acids, the hydrocarbon chain has only single carbon-to-carbon bonds. Hydrogen atoms complete the valence requirements, thus saturating the chain.
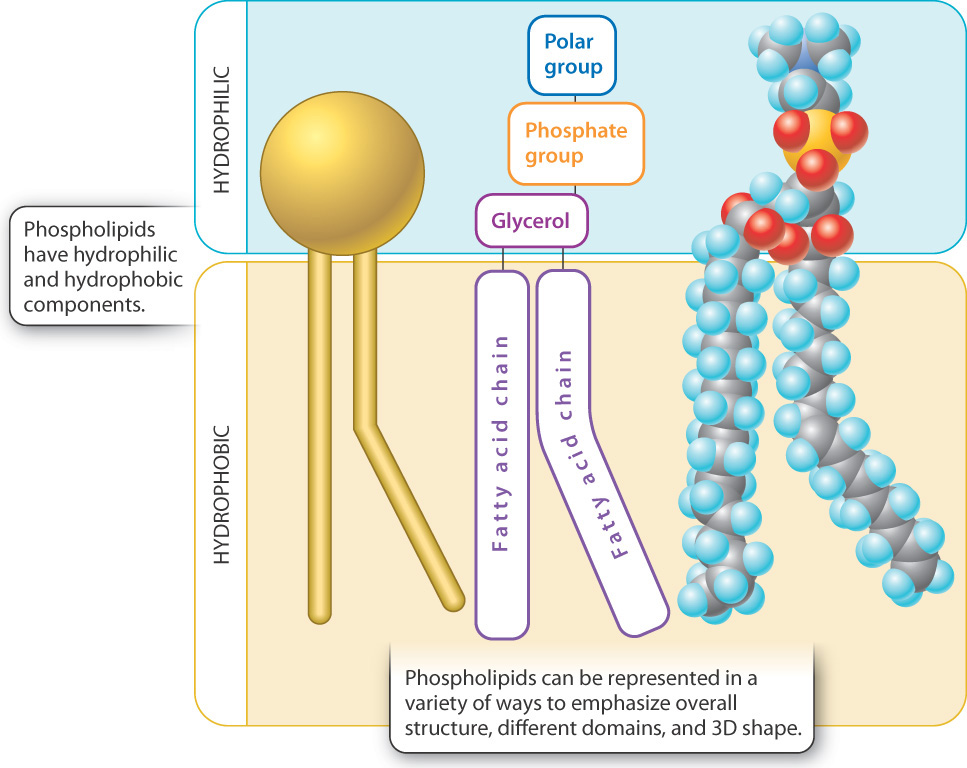


Saturated fatty acids are rigid and straight, and they are solid or semisolid at room temperature. ....as opposed to unsaturated fatty acids.
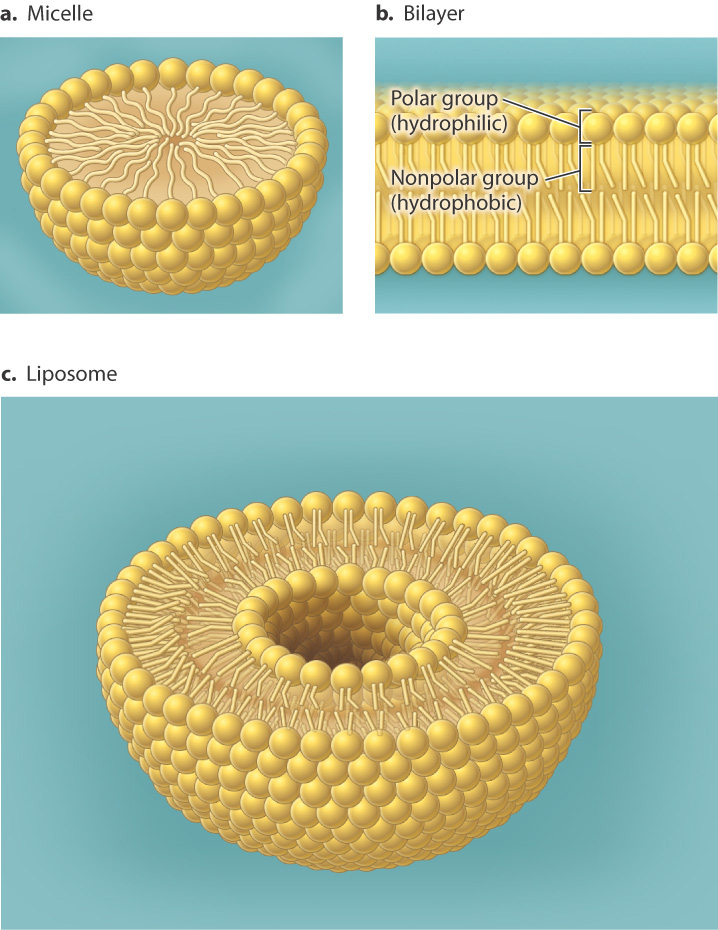
Thus, lipids play a crucial role in living cells: separating regions with different concentrations of ions and other chemicals, allowing the formation of that most basic of all Life units.....The Cell, with the lipids defining the rather critical celullar feature.......the cell membrane(s).
It is the Cell that takes the instructions found in DNA to make protein, and it is the proteins that are able to erearrange the carbohydrates to build and break down cellular structures to store or gain enegrgy for the cell and to synthesize the DNA.
It also takes a series of protein-enzyme catalyzed reactions to make lipids, for without thr lipid membranes, the compartmentalization necessary for the synthesis of the cellular structures, DNA and other lipids would not exist. Moreover, without an existing membrane, new lipid and membrane-associated proteins cannot organize themselves into functional attributes of the cell.
The cell theory states that all organisms are composed of cells, and all cells come from pre-existing cells. It is the basis for studying all life, whether single-celled or multicellular.
All cells are surrounded by a plasma membrane composed of the all important very hydrophobic lipid bilayer, which provides a fluid, but structured environment for a number of proteins floating within it, and protruding from it (See Mozaic Model).
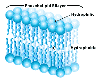
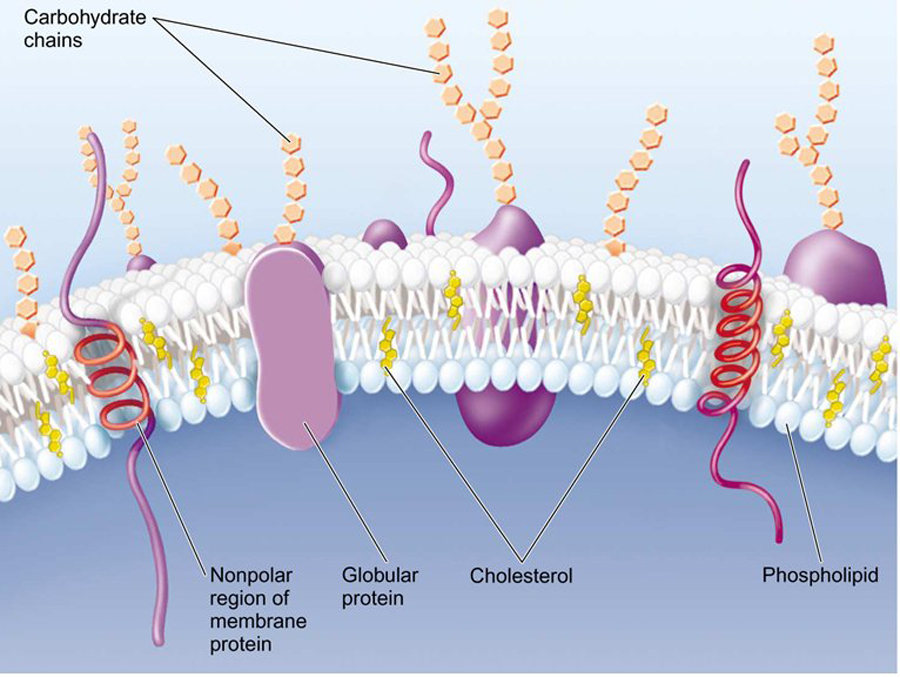
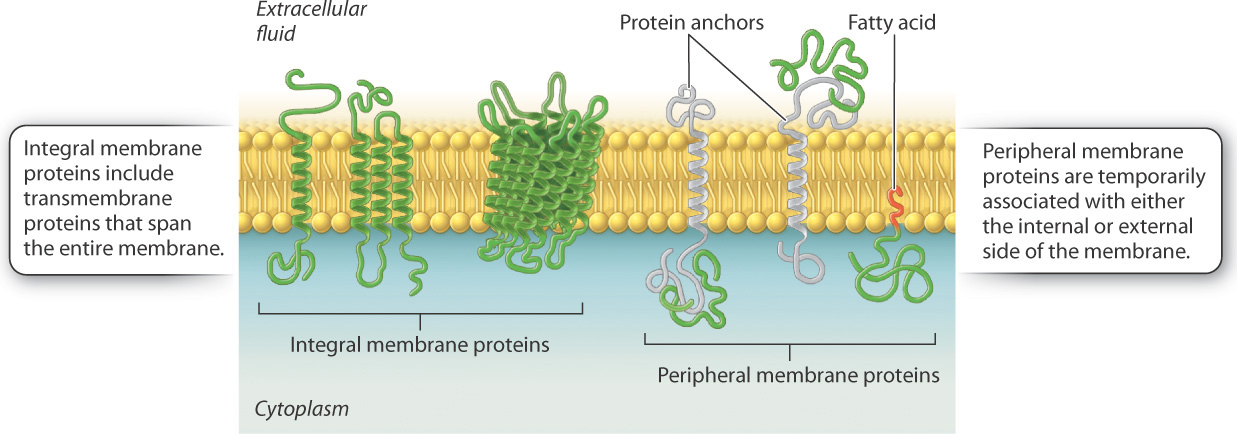
Some of these proteins traverse the membrane, with one part exposed on the inner cytoplasmic side and the other on the outer face of the cell.
Consequently, the plasma membrane acts as a selectively permeable barrier; allowing only a few substances to diffuse in and out of the cell.
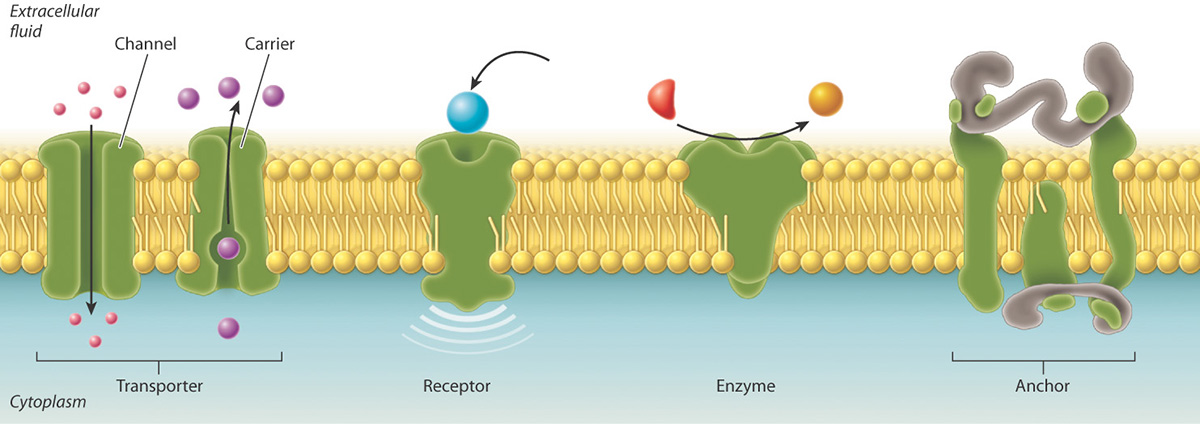
The membrane is peppered with assorted proteins, which provide a variety of means of transport specifically and non-specifically and in an activ or passive manner!
The plasma membrane also provides an interface with the outside world, where information can be received from adjacent cells and extracellular signals.
The membranes allow the cell to maintain a rather constant internal environment as well as separate and keep chemical and structural environments quite distinct.
As we have discussed previously, cell shape is often determined by size of cells, which is important for a number of reasons, but predominantly to effectively utilize function of the cell along with maintaning an appropriate surface area-to-volume ratio

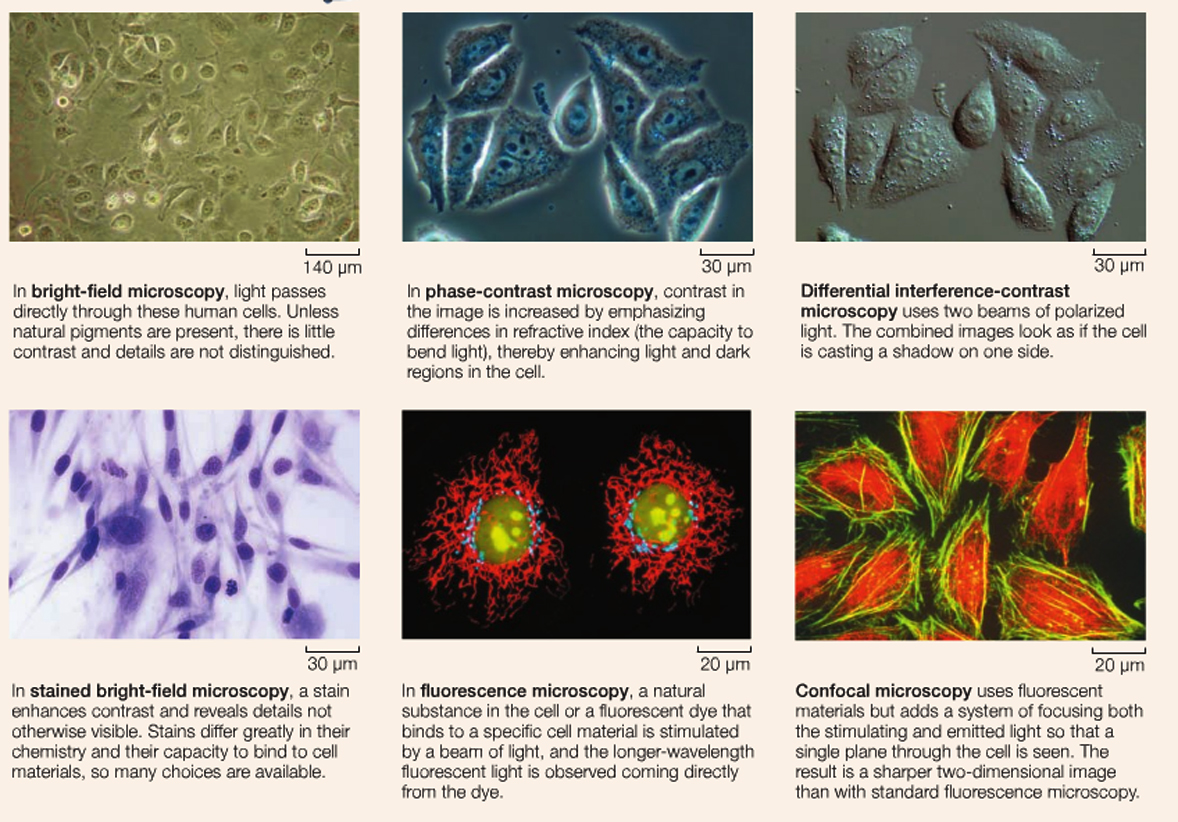
But what about the constituent parts of the cells.
As you already know living organisms can be classified into one of two major categories based on where, within the cell, the most genetic material is stored.
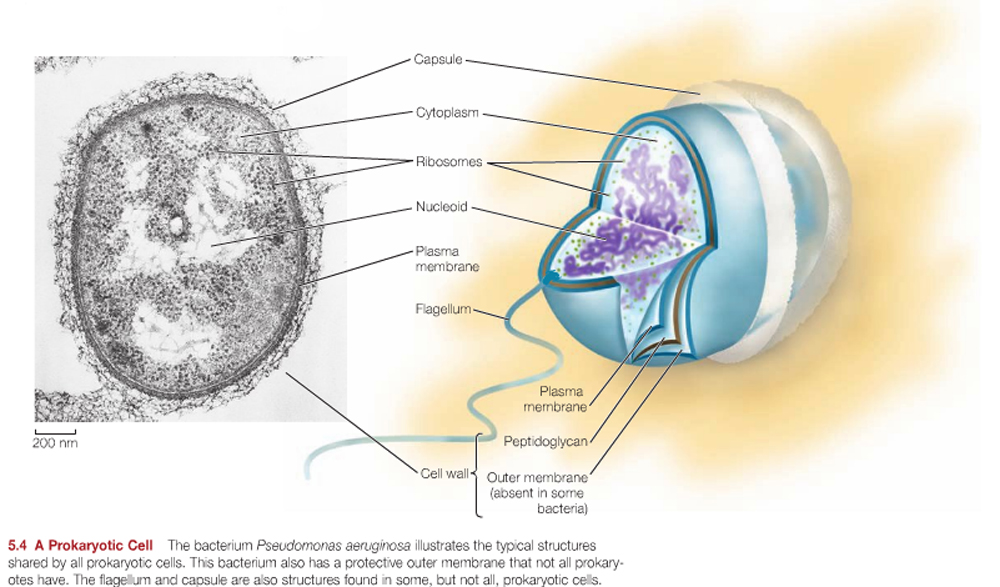
Remember, prokaryotes have no nucleus or other membrane-bounded compartments, within them (a generalization, which is not quite true for some bacteria.....). All this is in marked contrast to the eukaryotes.
The Prokaryotic Cell
Prokaryotes (along with the archaebacteria) inhabit the widest range of environmental extremes.
They can be found living at temperatures above boiling at thermal vents deep in the ocean. They also occur in extremely salty environments.
Some have been found deep in Earth's crust, far away from the sun, photosynthesizing organisms, and oxygen. These prokaryotes use inorganic, reduced chemicals for an energy source.
All prokaryotic cells share certain features:
All have a plasma membrane.All have a region called the nucleoid where the DNA is concentrated.The cytoplasm, is a densely packed soup (think of a Marta train at the height of rush hour - in 3D) consisting of the nucleoid, ribosomes and ALL other proteins/crabohydrates and some lipids and a liquid portion called the cytosol.
The Eukaryotic Cell:
Animals, plants, fungi, and protists have a membrane-bounded nucleus in each of their cells and are classified as eukaryotes.
Compartmentalization is the key to eukaryotic "higher" cell function.
The subunits, or compartments, within eukaryotic cells are called organelles.
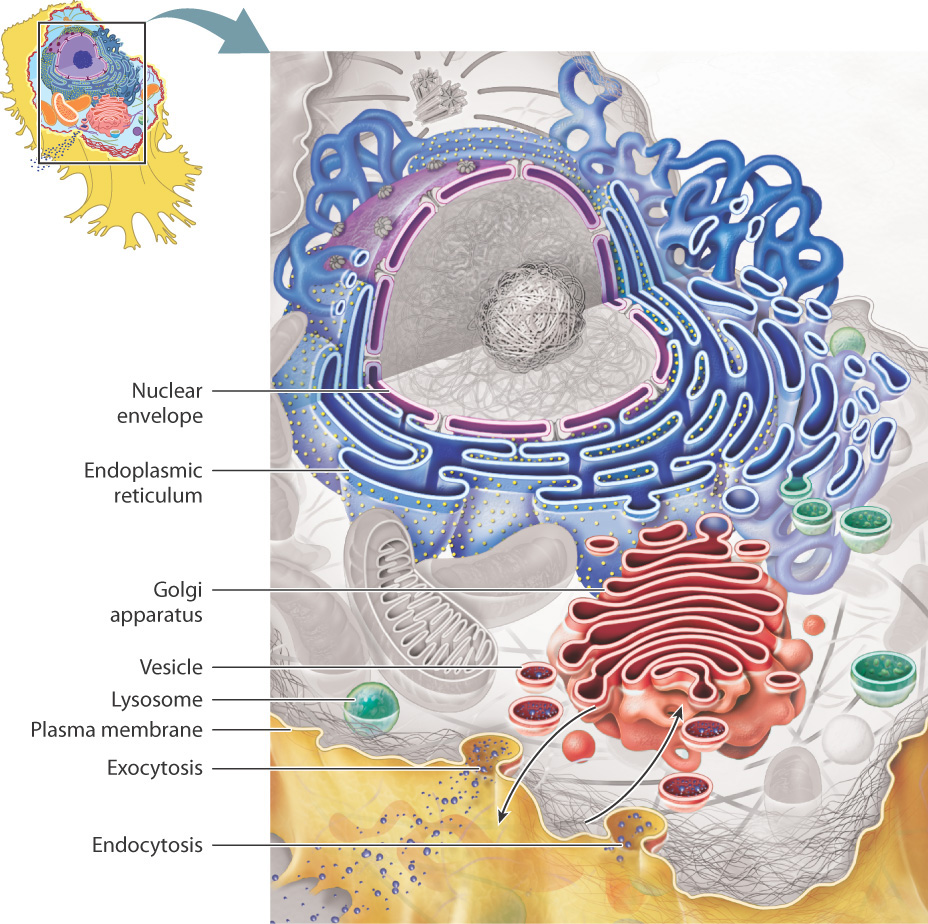
The nucleus contains most of the cell's genetic material (DNA).
The mitochondrion is the power plant and industrial park of the cell in that it is the major source of enfor the storage and conversion of energy.
The endoplasmic reticulum and Golgi apparatus make up distinct compartments where proteins are packaged and sent to appropriate locations in the cell.
The lysosome and vacuole are cellular digestive systems, where large molecules are hydrolyzed into usable monomers.
The chloroplast performs photosynthesis in bacterial and plant cells.
As you know, Eukaryotic cells tend to be larger than prokaryotic cells, and as such with all the volume changes and Volume/Surface Area ratio changes they have had to adapt a far more sophisticated network of support structures comprising the cytoskeleton, that provides shape and structure to cells, among other functions.



.jpg)
.jpg)
