- Home
Welcome !
Welcome to john houghton's home page for his biology courses. This site is designed as a hub for curating and sharing lectures, course syllabi, assignments, and links to relevant resources. Use the menu bar at the top of the screen to navigate through the site.
(Please note: this page is currently under construction.)

- BIOL 2107
Fall '22 CRN87989
Lectures: (1)
- Courses
- Resources
General Resources

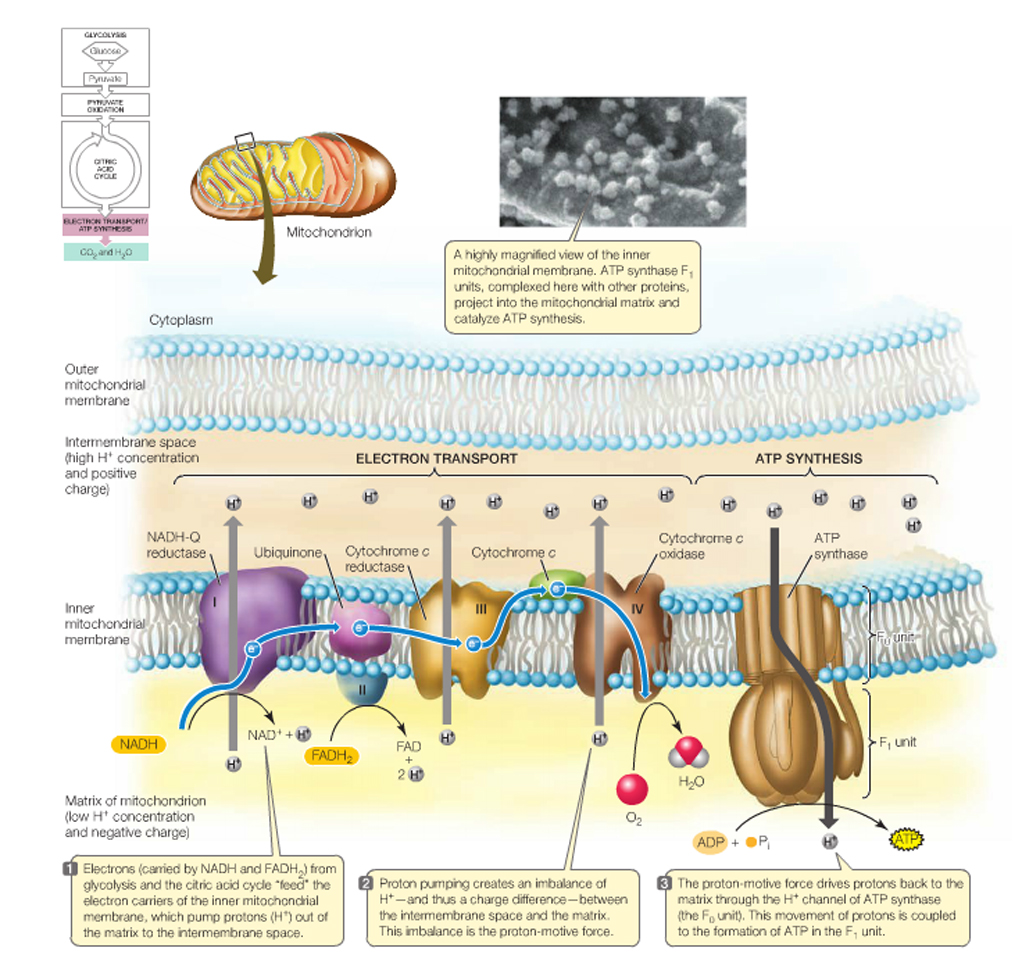
Respiratory Chain: in eukaryotes the repiratory chain of proteins is normally found in the mitochondrion, wheras in prokaryotes- the chain is found in the .....inner cell membrane.
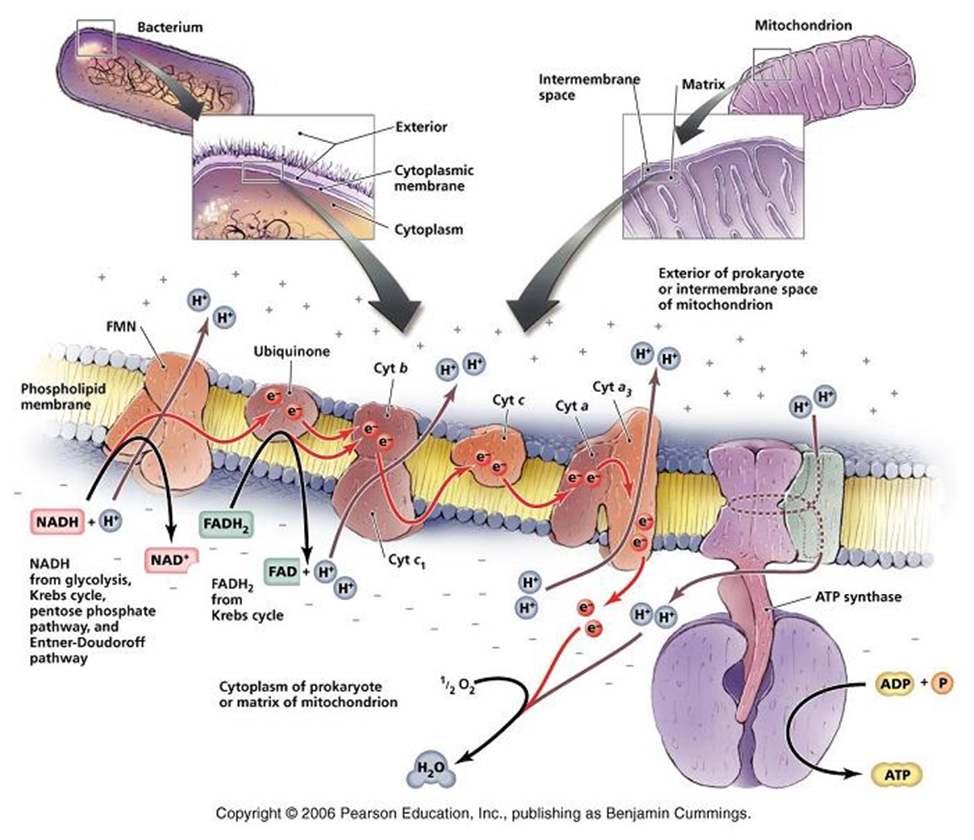
The respiratory chain, which is simply a series of proteins embedded within the membrane of mitochondria or the cytoplasmic membrane of prokaryotes, isthe final component of complete glucose oxidation, and is needed to make use of the generated reducing agents and the energy they possess.
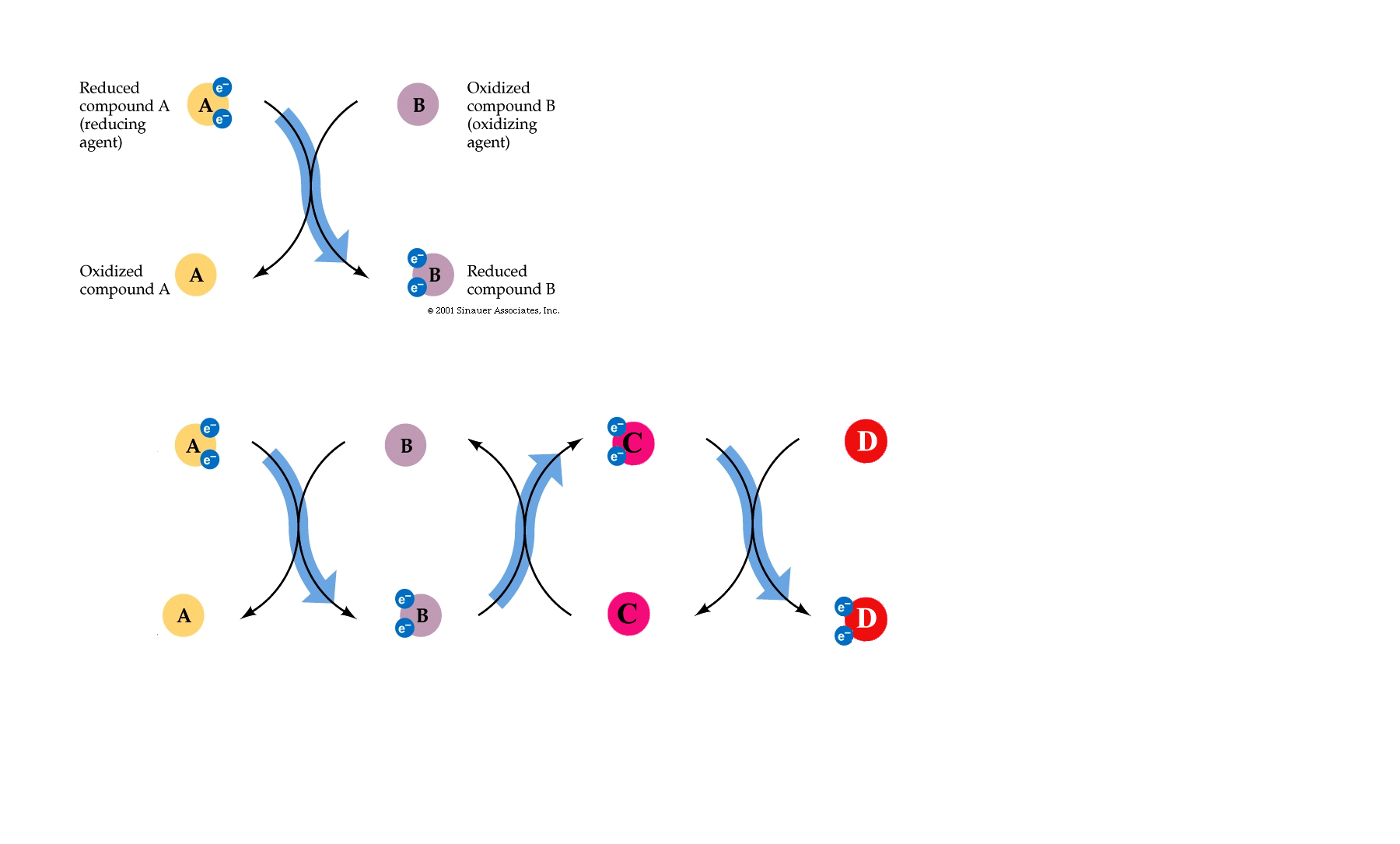
If the complete oxidation of NADH + H+ is allowed to proceed in short, decremental steps.....wherein a protein is reduced by NADH + H+ and the attached electrons- required to balance the charge of the reactants_followed by another protein being reduced by this newly oxidised protein......then, not only can the NAD+ of glycolysis and the citric acid cycle be "recycled", but the process can be repeated until more and more H+ 's can be exchanged with "carriers" from within the cell and the potential energy of the whole reaction can be harnessed and used to create ATP.
In this way electrons pass along a series of membrane-associated electron carriers called the respiratory chain.
The flow of electrons by way of a series of redox reactions causes the active transport of protons across the inner mitochondrial membrane and into the intermembrane space, creating a concentration gradient of H+ 's.
If these protons are then allowed to diffuse -through specific proton channels- back into the cell, down their concentration- (and electrical-) gradients, back into the matrix of the mitochondrion, then ATP can be created in the process through a special ATP generating turbine.
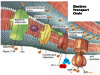
The entire process of ATP synthesis by electron transport through the respiratory chain is called oxidative phosphorylation
Thus, the respiratory chain transports electrons out of the and releases energy
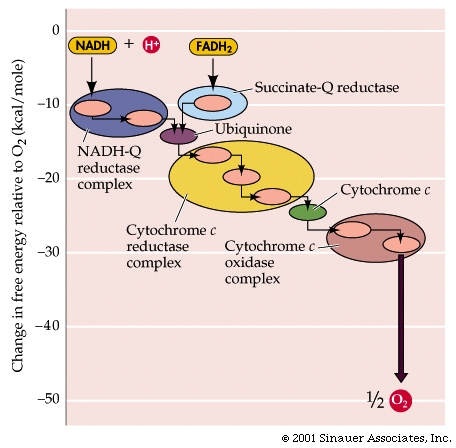
NADH + H+ passes its hydrogen atoms to the NADH-Q reductase protein complex. The NADH-Q reductase passes the hydrogens on to ubiquinone(Q) forming QH2.
The QH2 passes electrons to cytochrome c reductase, which in turn passes them to cytochrome c. Next to receive them is cytochrome c oxidase. The cytochrome oxidase passes them on to oxygen.
Reduced oxygen unites with two hydrogens to form water.
FADH2can also enter the chain through succinate-Q reductase.
As electrons pass through the respiratory chain, protons are pumped into the intermembrane space against a concentration and electrical gradient
The potential energy generated is called the proton-motive force.
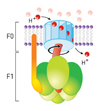
The movement of a proton through the channel causes the physical rotation of the core of the enzyme, which pushes the ADP and Pi so close together that they bond.
The synthesized ATP is transported out of the mitochondrial matrix almost as quickly as it is made, and is now available to the cell.
Contrasting Energy Yield:

A net total of 38 ATP molecules can be generated from each glucose molecule through glycolysis -followed by complete aerobic respiration.
The inner mitochondrial membranes of most mitochondria are impermeable to NADH. Consequently, to get into the matrix of the mitochondria energy is required (approximately one ATP for each of the two NADH2+ produced per molecule of glucose). This additiona expenditure of energy) reduces the net yield somewhat.
In contrast, fermentation has a net yield of only 2 ATP molecules from each glucose molecule.
The end products of fermentation (such as lactic acid and ethanol) contain much more unused energy than the end products of aerobic respiration.
In aerobic respiration, each NADH + H+ generates three ATP molecules, and each FADH2 generates two ATP molecules when consumed in the electron transport chain.
Coupled with
glycolysis, aerobic respiration captures ~ 63 percent of the
energy stored in glucose; fermentation captures
only ~ 3.5 percent. Consequently, Aerobic respiration is 18 times more efficient at harvesting
energy from glucose.
Metabolic Pathways.... It is no accident that glucose metabolism plays a central role in all metabolic pathways...

Glucose utilization pathways can yield more than just energy. They are interchanges for diverse biochemical traffic.
Intermediate chemicals are generated that are substrates for the synthesis of many macromolecules... lipids, amino acids, nucleic acids, and other biological molecules.

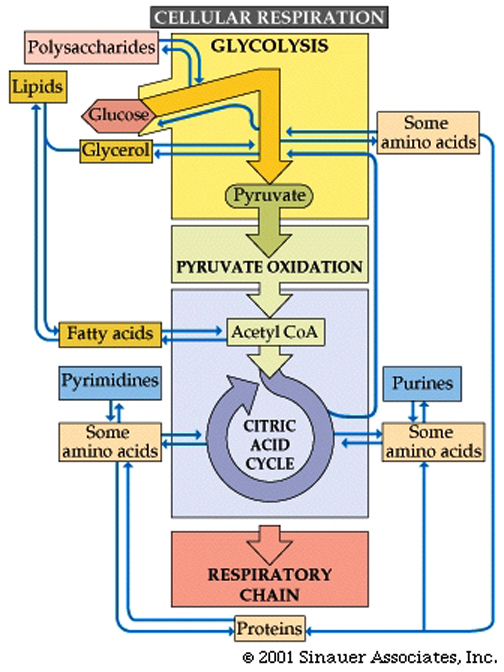
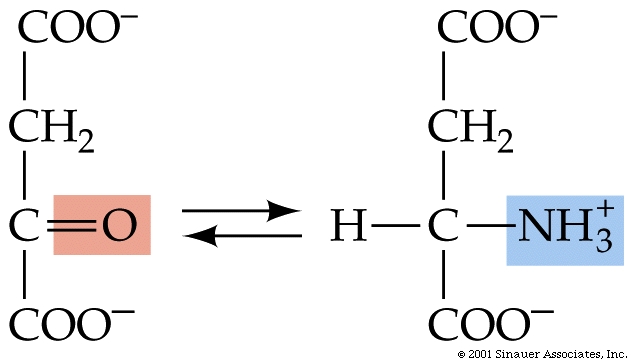
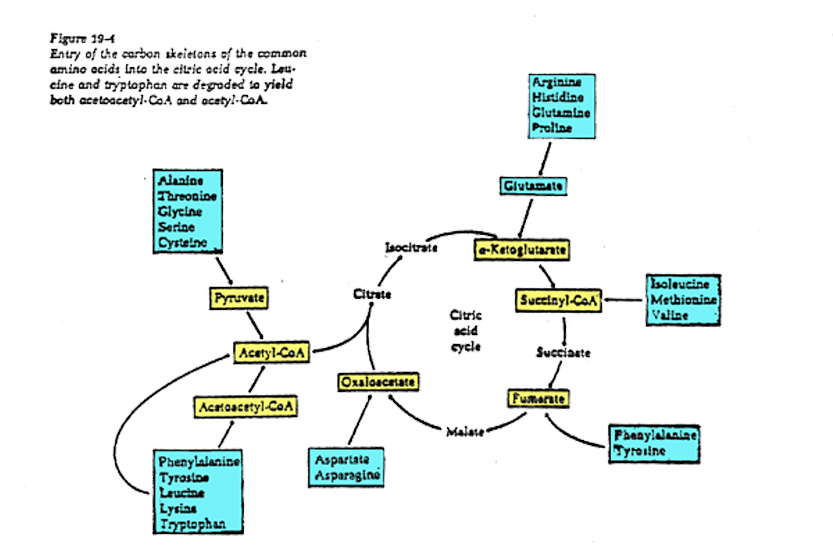
Catabolism and anabolism involve interconversions using "carbon skeletons" where, for example, the carbon skeleton of Oxaloacetate can be transformed (through a process called transamination) into the amino acid, aspartic acid -see figure below, which can then be uused to build (anabolise) peptides and nucleic acids.

Catabolic uses of molecules include the following:
Polysaccharides are hydrolyzed into sugars, which pass on to glucose for glycolysis.Lipids are converted to fatty acids, which become acetate (and then acetyl CoA), and glycerol, which is converted to dihydroxyacetone phosphate, an intermediate in glycolysis.Proteins are hydrolyzed into amino acids, which feed into glycolysis or the citric acid cycle.
Anabolic interconversions include the following:Gluconeogenesis is the process by which intermediates of glycolysis and the citric acid cycle are used to form glucose.Acetyl CoA can form fatty acids. Amino acids can be polymerized into proteins.Common fatty acids have even numbers of carbons because they are formed by adding two-carbon acetyl CoA units.The citric acid cycle intermediate alpha-ketoglutarate is the starting point for the synthesis of purines. Oxaloacetate is a starting point for pyrimidines.
Thus, catabolism and anabolism are interwoven
The levels of the products and substrates of energy metabolism are remarkably constant.
Cells regulate the enzymes of catabolism and anabolism to maintain a balance of molecules in the cell.
What happens if inadequate fuel molecules are available?
Polysaccharides are an intermediate storage form of energy. A typical person has about one day's worth of energy stored in the form of the carbohydrate "glycogen".
A typical person has about one week of needed amino acids stored as protein, and over a month's energy stored as fats.
Fats are compact energy storage molecules because they exclude water and are particularly hydrocarbon rich.
If food is withheld, glycogen is used up first, then fats. As fats are depleted, in humans this leads to a build up of ketones in the body, which develops into a condition called "ketosis "which elicits a mild euphoric state, and dulls the senses... as in a diabetic shock.
Fats cannot cross the blood-brain barrier. To supply glucose to the brain, glucose must be synthesized by gluconeogenesis (which is a reversal of glycolysis). This invariably requires the use of amino acids.
After fats are depleted, proteins alone provide the energy.
At this point, in humans at least, the important disease-fighting proteins (including antibodies) are also consumed, and the likeliness of severe illness increases.
or, the more accurate story......
All molecules absorb electromagnetic radiation, but differ in the specific wavelengths absorbed.
Molecules that absorb wavelengths in the visible range are called pigments.
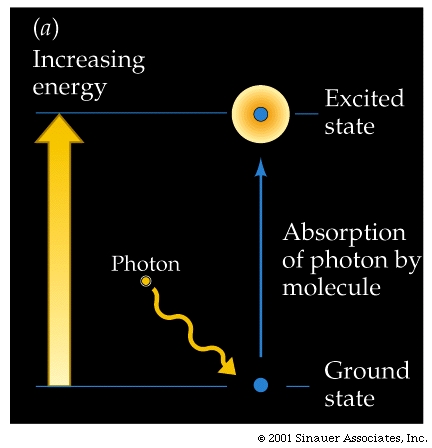
When a photon and a pigment molecule meet, one of three things happens:
The photon may bounce off the molecule (reflection), the photon may pass through the molecule (transmission), the photon may be absorbed by the molecule (excitation).
When it is absorbed, the photon disappears, but the energy can be used to raise the molecule that has absorbed the light from its energetic "ground state" to an excited state of higher energy. More specifically, the light energy causes some electrons break out from their normal orbital into higher orbitals, making them more likely to leave the confines of the atom, molecule.
The difference between the "excited" and the ="ground state" is precisely equal to the energy of the absorbed photon.

Photosynthetic organisms use chlorophylls (along with other accessory pigments) where the Chlorophylls are the most important pigments in photosynthesis.

Plants have two predominant chlorophylls: chlorophyll a and chlorophyll b, which differ slightly in their structure.
Even so, both have a similar ring structures.
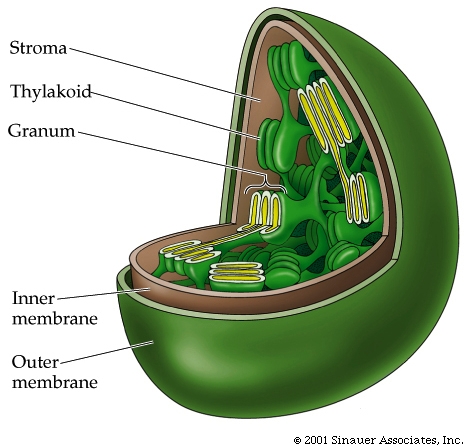
In the center of the chlorophyll ring is a magnesium, which coordinates with the chlorophyll ring enhancing fluidity of electron flux within the ring. At the peripheral location of the ring is a long hydrocarbon tail that can associate with the hydrophobic region of the thylakoid membrane in which it is found.
These chlorophylls absorb blue and red wavelengths of light, which are near either end of the visible spectrum.
Other accessory pigments, such as the carotinoids; b-carotene, also absorb photons that are intermediate in energy between the red and blue wavelengths, and then transfer a portion of that energy to the chlorophylls.
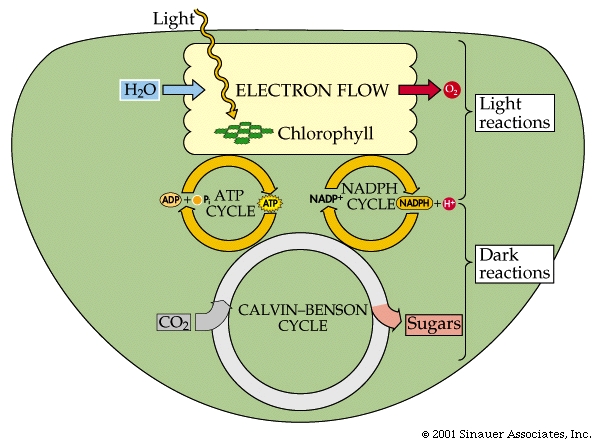
LIGHT Reaction: Light Absorption
As mentioned previously, when a pigment molecule absorbs a photon it enters an "unstable" excited state.
One of two things can now happen.
The molecule can return to it's ground state, emitting much of the absorbed energy as fluorescent energy. When the molecule fluoresces, it emits a photon fluorescence that is of a longer wavelength (less energy) than that which induced the orginal excitation.
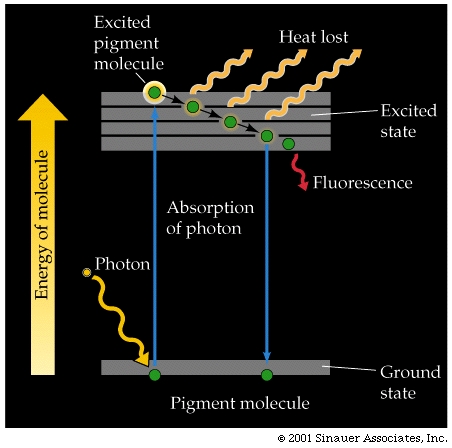
An alternative to simple fluorescence is where the excited molecule can also pass some of the absorbed energy on to other pigment molecules.
Pigments in photosynthetic organisms are arranged into an energy-absorbing antenna systems, allowing a build up of energy and relatively increased efficient of transfer from the photon, enhancing the potential for one chlorphyll to become hyper excited and lose an electron.
Excited chlorophyll (Chl*) now acts as a reducing agent
The reducing capability of Chl* is increased because excited molecules have electrons zipping around farther away from the nucleus.
These less tightly bound, highly energized electrons are more likely to be passed on to other "less energized" molecules in redox reactions to another oxidizing agent, as we saw with the electron transport chain in the membranes of prokaryyotes and eukaryotes during "respiration". When this happens, chlorophyll becomes a positively charged ion, which is NOW MISSING an electron.......
Electron Flow, Photophosphorylation, and Reductions


The electrons flow through a series of carriers, where redox reactions occur one after another, and the energy of these redox reactions is used to pump protons...(deja vu?). This process is referred to as photophosphorylation.
There are two different systems for flow of electrons in photosynthesis -Cyclic and Non-Cyclic.
For the sake of simplicity, we will mainly discuss Non-Cyclic systems, which produce most, if not all the oxygen in the atmosphere, as well as NADPH + H+ and ATP.
The oxidized chlorophylla is reduced by an electron from a manganese-containing protein called Protein Z
The protons (H+) that are generated are then released into the thylakoid lumen, where they help to build a proton gradient.
In summary, NON-CYCLIC electron flow effectively takes one molecule of water, four "photons", one molecule each of NADP+ and ADP and one Pi to make one molecule each of NADPH+ H+ and ATP as well as one half a moleaule of that all important product.. O2.
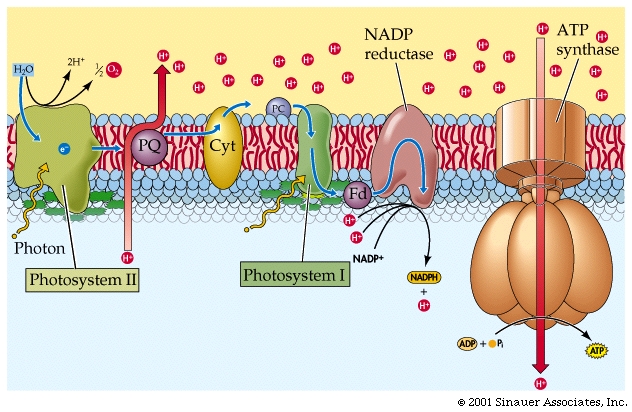
The energy captured in the light reactions is stored in ATP and NADPH+ H+ to drive the Calvin-Benson cycle, which uses more ATP than NADPH + H+.
In CYCLIC electron flow, photosystem II acts on its own, without the need for photosystem I. There is also NO use of WATER and does not make NADPH2
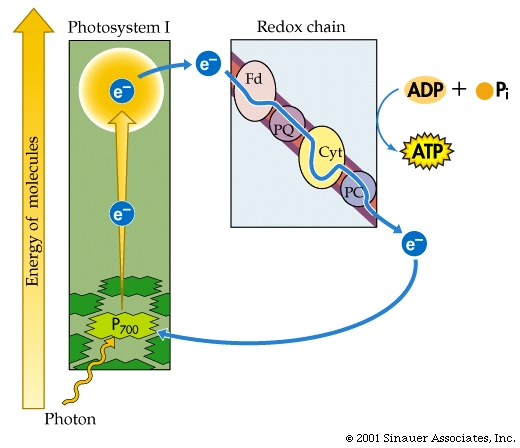
In summary,CYCLIC electron flow takes photons through a special chlorophyll molecules, passing excited electrons through a redox chain to produce ATP and some free energy as heat. The, now electron-deficient chlorophyll has the electrons restored, and the process repeats. This form of photosynthsis is thought to be the more ancestral form of photosynthesis and is presently found in ANOxygenic photosynthetic bacteria (purple bacteria). Cyanobacteria, like plants undertake the OXYgenic photosynthesis, with TWO photoreaction cells/systems
The Dark Reaction.... better know as the Calvin-Benson cycle, which is composed of three processes to reduce CO2 to carbohydrate (the last part of the photosynthetic equation referenced earlier).
Fixation of CO2, by combination with RuBP (this is catalyzed by an enzyme called RuBisCO, which is THE most common enzyme in all the world).
Conversion of fixed CO2 into carbohydrate (G3P). This step uses ATP and NADPH2+.
Regeneration of the CO2 acceptor RuBP by additional ATP.
The end product of the cycle is glyceraldehyde 3-phosphate, G3P, which is a three-carbon sugar-phosphate, also called triose phosphate.


There are two fates for the G3P that is produced:
~ One-third ends up as STARCH, which is stored in the chloroplast and serves as a future source of... you geiessed it, glucose.
~ Two-thirds is converted into glucose directly to provide a source of energy for generic cellular energy.... I wonder how that works......?
Most of the stored energy is released by the last part of glycolysis and cellular respiration by the plant itself, during growth, development, and reproduction.
Don't forget, some of the carbons of glucose can also be used the carbon skeletons for amino acids, lipids, and nucleic acids.

Some of this stored energy is consumed by heterotrophs, where glycolysis and respiration release the stored energy.