- Home
Welcome !
Welcome to john houghton's home page for his biology courses. This site is designed as a hub for curating and sharing lectures, course syllabi, assignments, and links to relevant resources. Use the menu bar at the top of the screen to navigate through the site.
(Please note: this page is currently under construction.)

- BIOL 2107
Fall '22 CRN87989
Lectures: (1)
- Courses
- Resources
General Resources

Review: In the last couple of lectures we discussed the important basic aspect of chemistry, as they relate to Biological systems, and how the building up of biological macromolecules, Carbohdrates, Proteins, Nucleic acids and Lipids involves the removal of water (although lipids don't officially form "true" macromoleculess and how some of these macromolecules can be used to do work in the cell (proteins -enyzmes, catalysis; carbohydrates -storage in the form of starch, glycogen).
We also discussed the ability of Eukaryotic cells to be much larger than prokaryotic cells and, as such, overcome the surface with all the volume changes and Volume/Surface Area difference they have had to adapt a far more sophisticated network of support structures comprising the cytoskeleton, that provides shape and structure to cells, among other functions.
We then revisited and contrasted the cellular structures of different generic life forms, the "simple" prokaryote cell and the larger, "structurally more complex" eukaryotes -animals and plant -all of which can utilize these different macromolecules in similar, but different ways.
The Eukaryotic Cell:
Animals,plants, fungi, and protists have a membrane-bounded nucleus in each of their cells and are classified as eukaryotes.
Compartmentalization is the key to eukaryotic "higher oorder" of cellular function.
The subunits, or compartments, within eukaryotic cells are called organelles

The nucleus contains most of the cell's genetic material (DNA).
The mitochondrion is the power plant and industrial park of the cell in that it is the major source of enfor the storage and conversion of energy.
The endoplasmic reticulum and Golgi apparatus make up distinct compartments where proteins are packaged and sent to appropriate locations in the cell.
The lysosomes and vacuoles are cellular "digestive systems", where large molecules are hydrolyzed into usable monomers.
And, finally, the chloroplast performs photosynthesis in bacterial and plant cells.
So, having discussed, somewhat, the building blocks and how these are potentially utilized for different cell-types, lets go back to the first lecture again. when we introduced the notion that ALL life foms live and die subject to all the Laws of chemistry and PHYSICS. We briefly discussed the importance of energy to living cells, and that,according to the laws of physics, this energy can neither be created nor destroyed...but it can be changed into different forms, kinetic and potential energy, and that when it is converted between these forms some of the energy (while not "destroyed", per se) becomes unuseable in the form of HEAT.
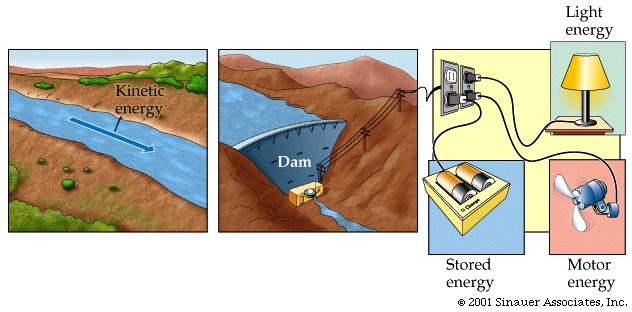
In discussing heat and energy levels we can now introduce the notion that some molecules have an inherent store of energy, in the form of "free energy" and that chemical reactions, which involve these molecules, can be either exergonic (give off energy -some of which can be utilized) or endergonic (require the expendititure of useful energy to allow reactions to proceed).
Well, in the next few lectures I would like to try to bring all these various aspects of life, macromolecules, energy and living organisms together and talk about just how these organisms are able to survive in the world by "harnessing" the energy from the environment and converting this rich energy source of "potential energy" to be able to do work within the living cell.
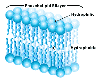
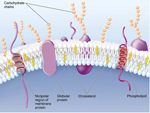
As noted, cells are surrounded by a plasma membrane composed of the all important very hydrophobic lipid bilayer, which provides a fluid, but structured environment for a number of proteins floating within it, and protruding from it (See Mozaic Model).
Some of these proteins traverse the membrane, with one part exposed on the "inner" cytoplasmic side and the other on the "outer" face of the cell.
Consequently, the plasma membrane acts as a selectively permeable barrier; allowing only a few, choice substances to diffuse in and out of the cell.
The membrane is peppered with assorted proteins, which provide a variety of means of transport specifically and non-specifically and in an activ or passive manner!
The plasma membrane also provides an interface with the outside world, where information can be received from adjacent cells and extracellular signals given shared.
The membranes allow the cell to maintain a rather constant internal environment as well as separate and keep chemical and structural environments quite distinct.
Prokaryotes and Mitochondria...
How do cells create energy? As discussed in much earlier on in this lecture series... they don't.
Biological systems follow the physical laws.....E = mc2, and as "energy is neither created nor destroyed"- they can only transform energy from one form to another?
In living cells energy transformations are primarily molecular movement and changes in chemical bonds, which are related to changes in matter.
Effectively, for biological systesms there are two main types of energ, which are kinetic and potential energy.
Potential energy is energy of state or position—it is stored energy. Potential energy is like money in the bank. It is there until it needs to be spent, which is when it becomes active or kinetic energy ("energy in action").
Kinetic energy provides the necessary means for the cell to undertake its work, alowing the cells to alter the energy states of motion or matter. Abusing the analogy of ATP and energy, still further- it allows the cell to spend the money that it had stored in the from of potential energy.

The FIRST law of thermodynamics: or the law of the conservation of energy, states: the increase in the internal energy of a system is equal to the amount of energy added to the system by heat, plus the amount added in the form of work done on the system.
Even though living cells are "open systems" (i.e. they exchange matter and energy with their surroundings), Even livingh organisms must obey these fundamental laws.
Conservation of energy and the characteristics of energy conversions apply to both closed and open systems of matter and energy.
First law of thermodynamics (reworded): During any inter conversion of forms of energy the total energy at the outset will equal the total final energy.
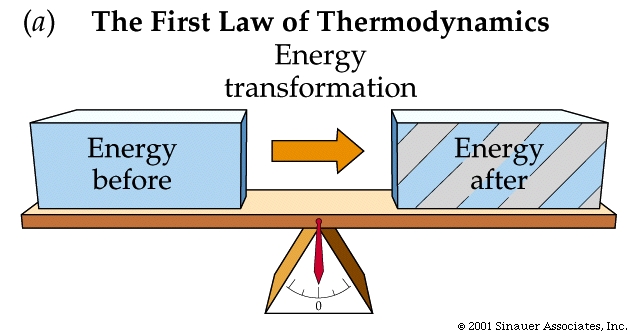
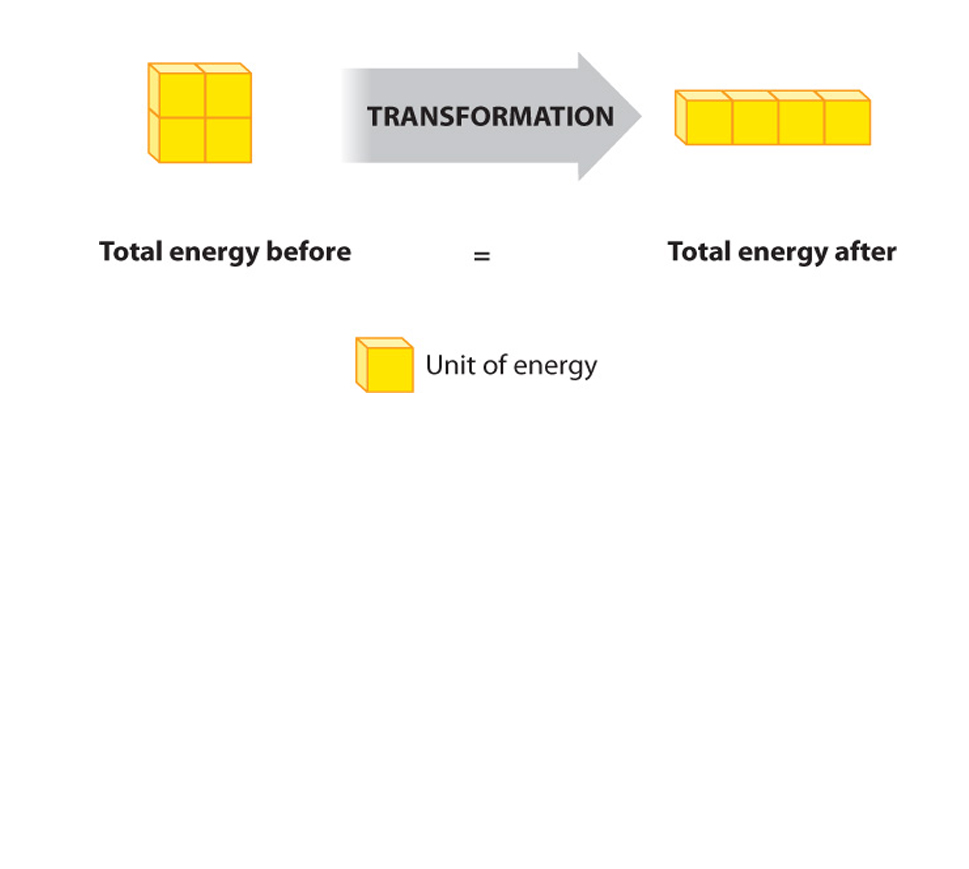
The SECOND law: the total entropy of any thermodynamically isolated system tends to increase over time, approaching a maximum value. Put another way- Not all energy can be used....., and its interconvesion promotes the fact that disorder tends to increase.....
As it applies to metabolism the second law of thermodynamics might be: When energy is being transformed, some is inevitably unavailable to do work (even though it is still present).
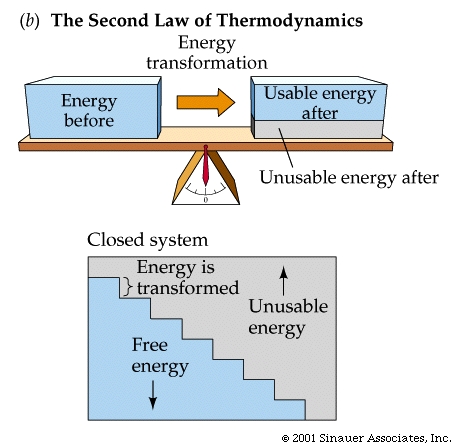
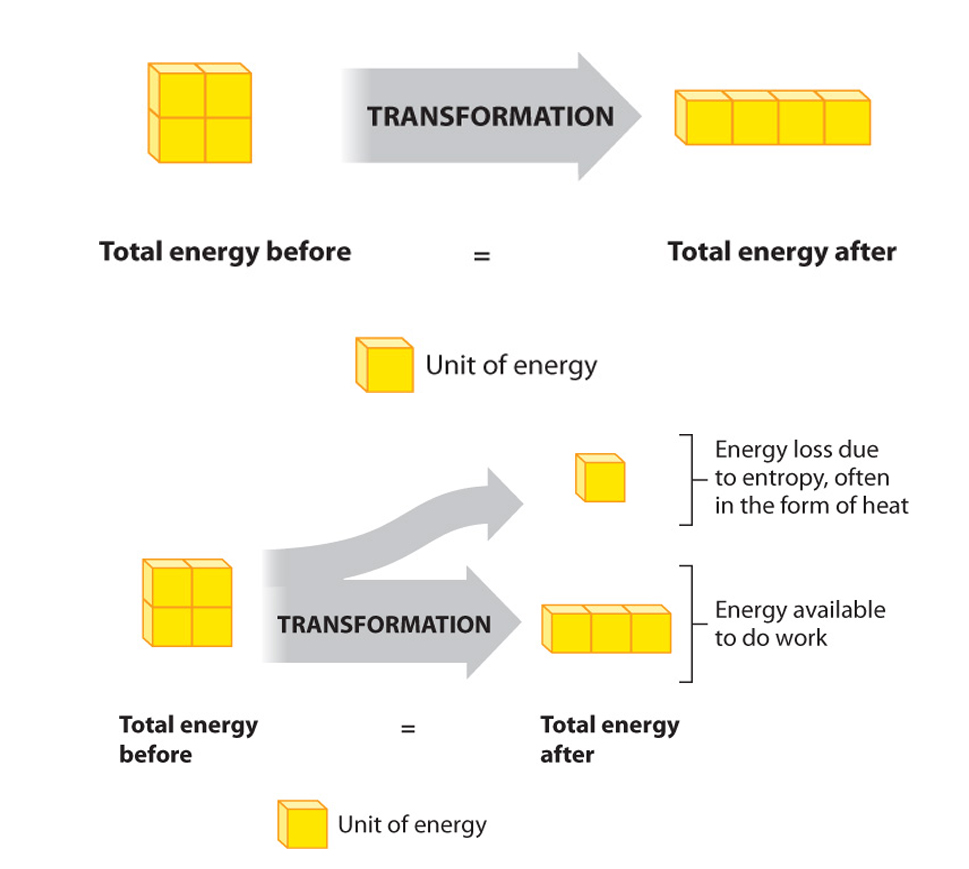
Theoretically, therefore, it is possible that conversion of energy is 100% efficient. Unfortunately, in the real world energy is never utilized perfectly, so the efficiency is always less than 100%.
Chemical reactions -just like any other reactions involving energy- either "consume" or "release" specific types of energy.
A spontaneous reaction goes more than halfway to completion without input of energy.
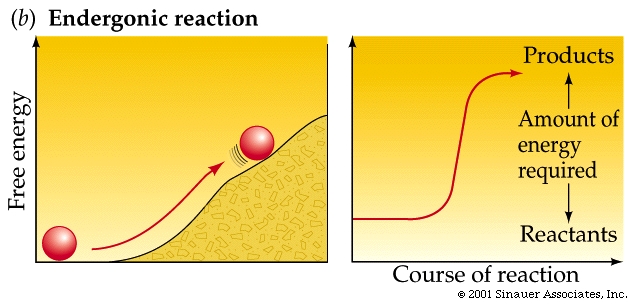
Non-spontaneous reactions proceed only with an input of energy.
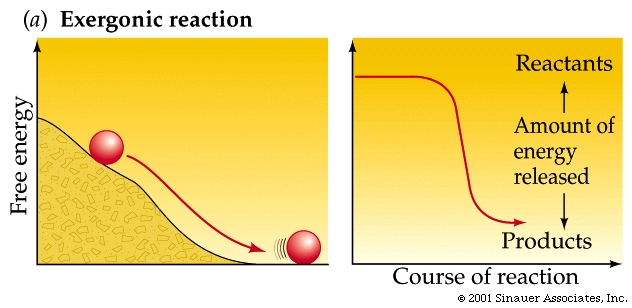
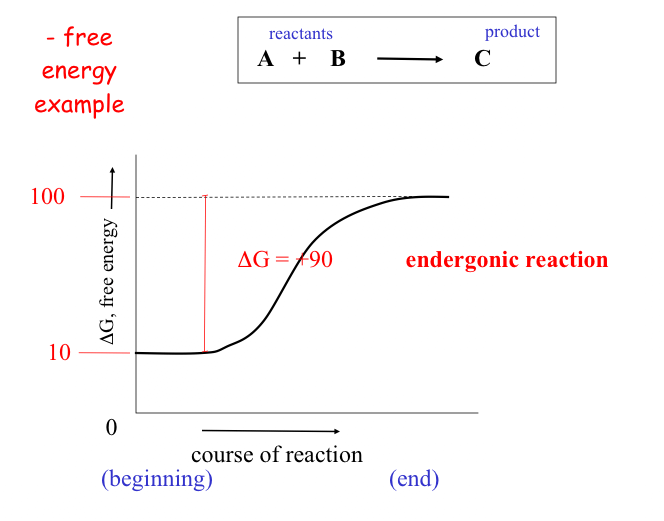
Spontaneous reactions invariably give off energy (often in the form of heat) and are called exergonic reactions These reactions have a negative DG values (they release energy).
Other reactions actually require energy and are thus called endergonic and have positive DG values. (they actually "consume" energy).
All reactions, however - at least in principle, are reversible. (A <---> B).
If (under certain, given conditions) A ----> B is spontaneous (and exergonic), then B ---->A must be less than spontaneous (and endergonic).
All other things being equal, adding more A generally speeds up the forward reaction,
adding more B would tend to favour (or speed up) the reverse reaction,
At some relative concentration of A and B, the net forward and reverse reactions taking place are in balance, and effectively cancel each other out. At this point, no further "net" change occurs. However, reactions of individual molecules still continue, thus creating a chemical equilibrium.
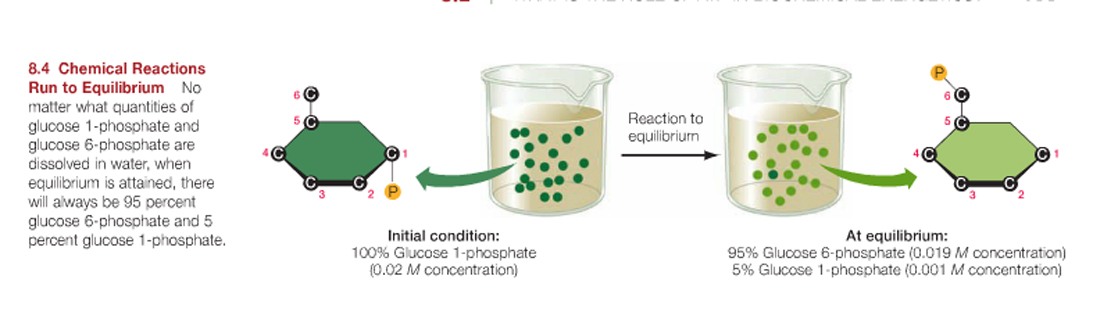
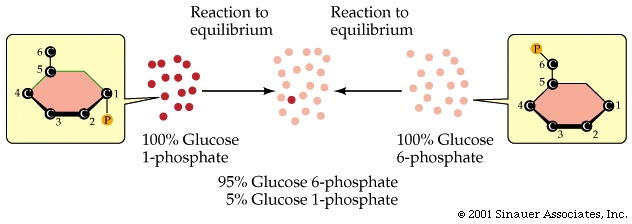
An example of just such a chemical equilibrium is when glucose 1-phosphate is converted in the cell to glucose 6-phosphate.
At pH 7 and 25oC, the equilibrium of reactant-to-product is 1:19, and so the forward reaction will effectively go to 95% completion before it reaches its equilibrium.
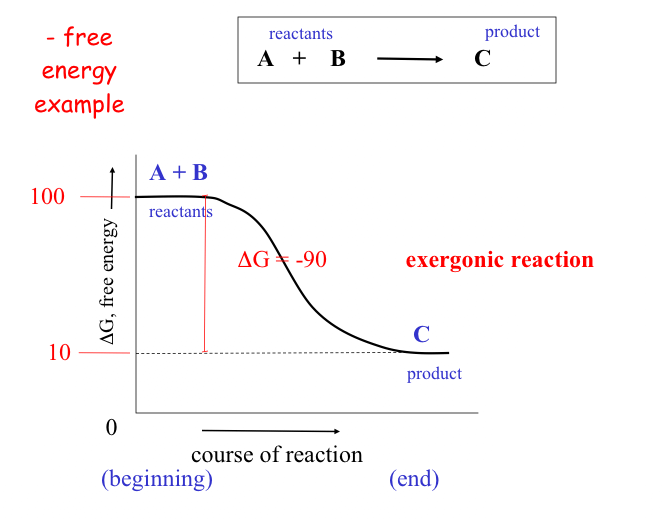
As a rule of thumb: the farther a reaction goes toward completion in order to reach equilibrium the greater the amount of "free energy" released.

Not too surprisingly, there is a direct relationship between the amount of energy released by a reaction (–DG) or the amount taken up (+DG) and the tendency for a reaction to run to completion without an input of energy.
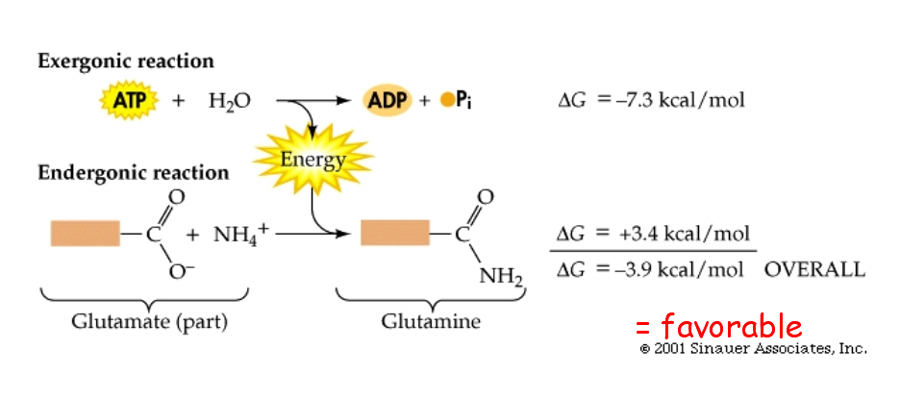
The above figure also demonstrates the interconvertability of energy from one resource to the next. While it is easy to predict the direction that a spontaneous reaction will go, it is not so easy to predict the "likelihood" or rate of the reaction.
For a reaction to proceed, an energy barrier often must be overcome......
An initial investment
of energy must be made -like the energy from a lightning strike, giving
rise to a forest fire.
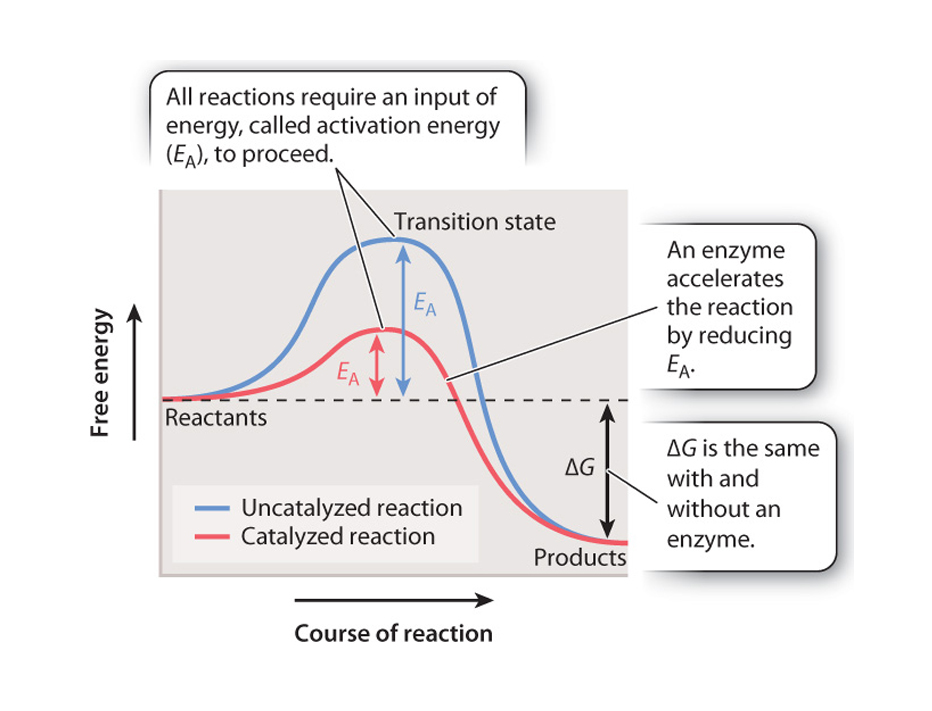
The energy that needs to be invested to initiate a reaction is called its activation energy.
All reactions have activation energy requirements, even extremely exergonic
ones.
Activation energy
is the energy needed to put molecules into a suitable "transition state", which is an inherently unstable state.
Transition-state species invariabley have higher free energy than either reactants
or products.....

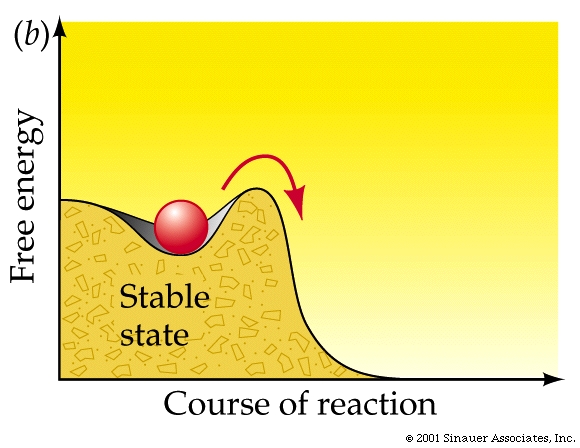

Adding enough heat to increase the average kinetic energy of the molecules is often how exergonic reactions are initiated (as in the case of the lightning strike).
In biological systems, adding heat is not always an appropriate way for biological systems to drive reactions.
Enzymes: Biological Catalysts
A catalyst is any substance that changes the rate of a chemical reaction -without itself being used up in the reaction.
Living cells use biological catalysts to increase rates of chemical reactions, called enzymes.
Most biological catalysts are proteins called enzymes -certain RNA molecules are catalysts and are called ribozymes.
Enzymes, acting as biological catalysts, solve this problem.
A catalyst does not cause a reaction to take place that could not take place eventually without it being present, but it does substantially lower the required energy of activation.
Enzymes are biological catalysts as they efffectively lower the activation energy barrier but DO NOT affect equilibrium, nor do they alter the difference in free energy between the reactants and the products, only the rate of the reaction.

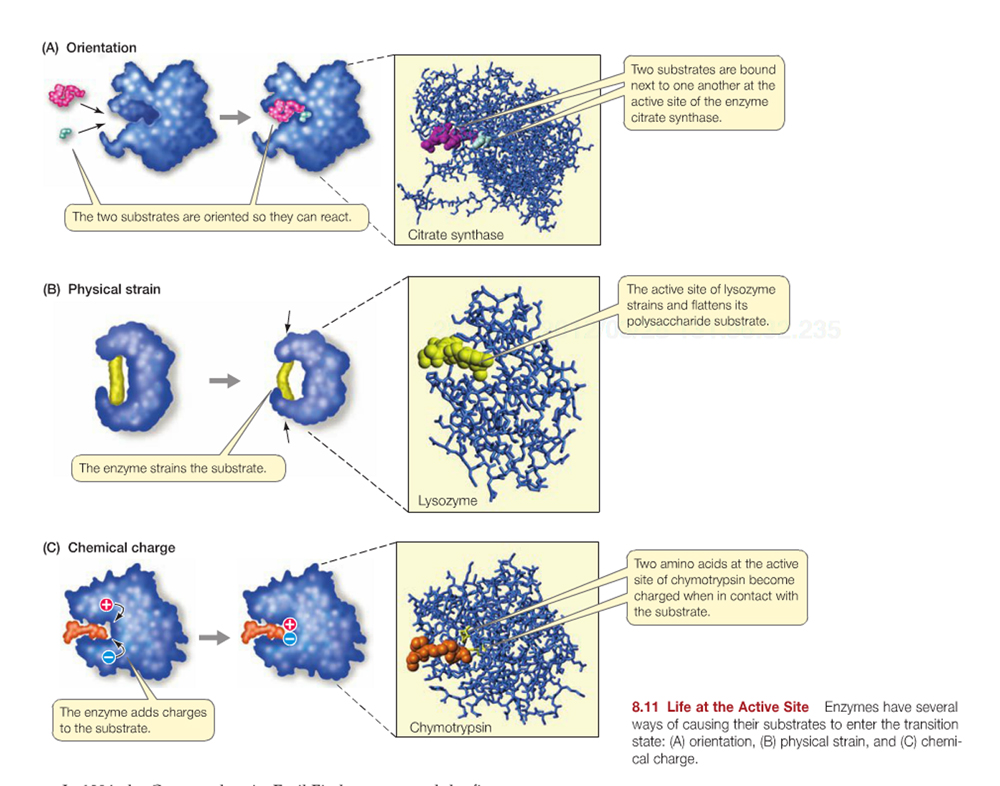
Enzymes exert their affect in a variety of ways through binding specific reactant molecules called substrates: (a) simply enhancing the orientation of reactant molecules, (b) inducing physical strain, or (c) inducing chemical changes in the substrate/reactants to enhance formation of charges intermediates.
Enzymes are highly specific. They bind specific substrates and catalyze particular reactions under certain conditions.
As such the activity of different enzymes can be modified / regulated by the reversible (or sometimes "irreversible") binding of other molecules to the enzyme either at the "active site" of the enzyme or at another site of the enzyme -termed "allosteric regulation".

A number of reactions within the cell often require additional "factors" that will often be used up during the course of a reaction.

The table above is a list of examples of cofactors, coenzymes,
and prosthetic groups.
Coenzymes must react with an enzyme, separate, and then participate in
other reactions.
ATP and ADP are coenzymes. They are
also substrates of the reactions.
So, how do cells go about using their enzymes to derive energy? Moreover, what are the general rules by which such energy production is defined?
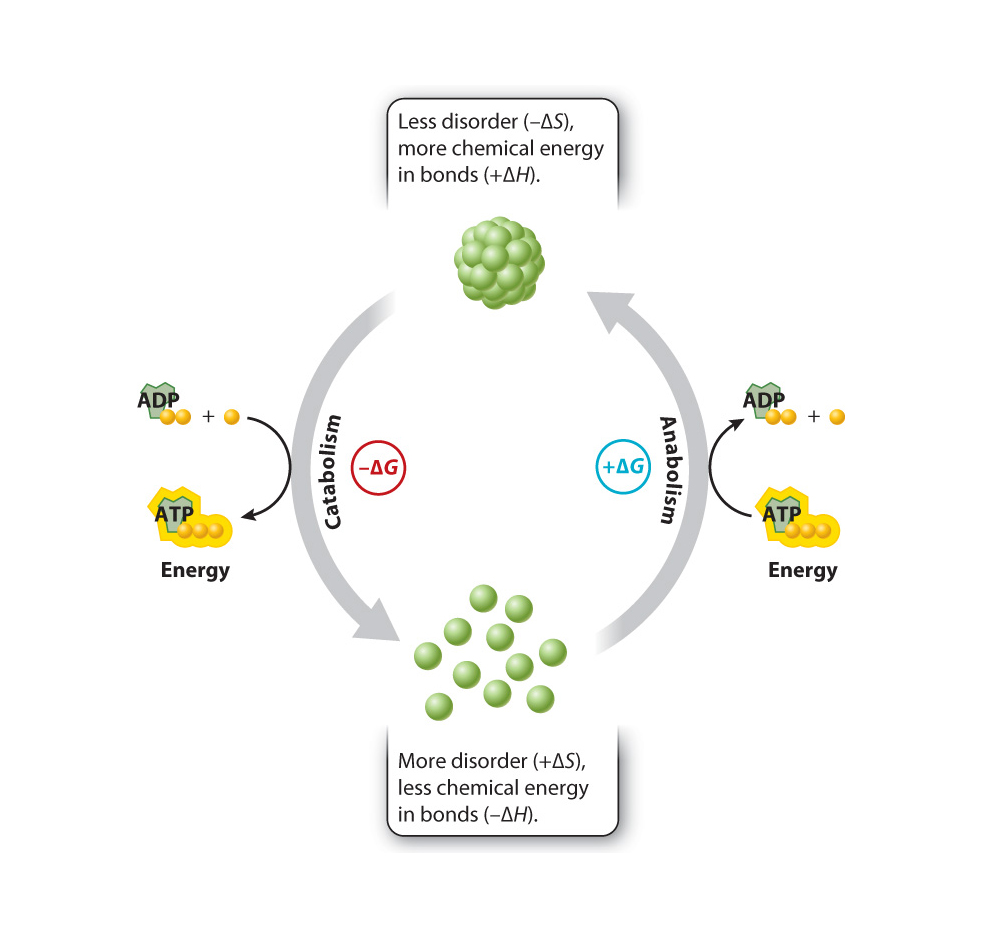
Metabolism can be divided into two types of activities: anabolic and catabolic reactions.
Anabolic reactions are those that link simple molecules together to make complex ones. These are energy-storing reactions......(e.g.condensation reactions?)
Anabolic reactions, which make single products from many smaller units; such reactions "consume" the useable energy.
Catabolic reactions may reduce an organized substance, such as a glucose molecule, into smaller more randomly distributed substances, such as carbon dioxide and water; such reactions generally "release" useable energy.
ATP is adenosine triphosphate, the universal source of useable energy in biological systems, and it can can be hydrolyzed to yield ADP and an inorganic phosphate ion (Pi, short for HPO42-).
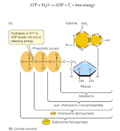
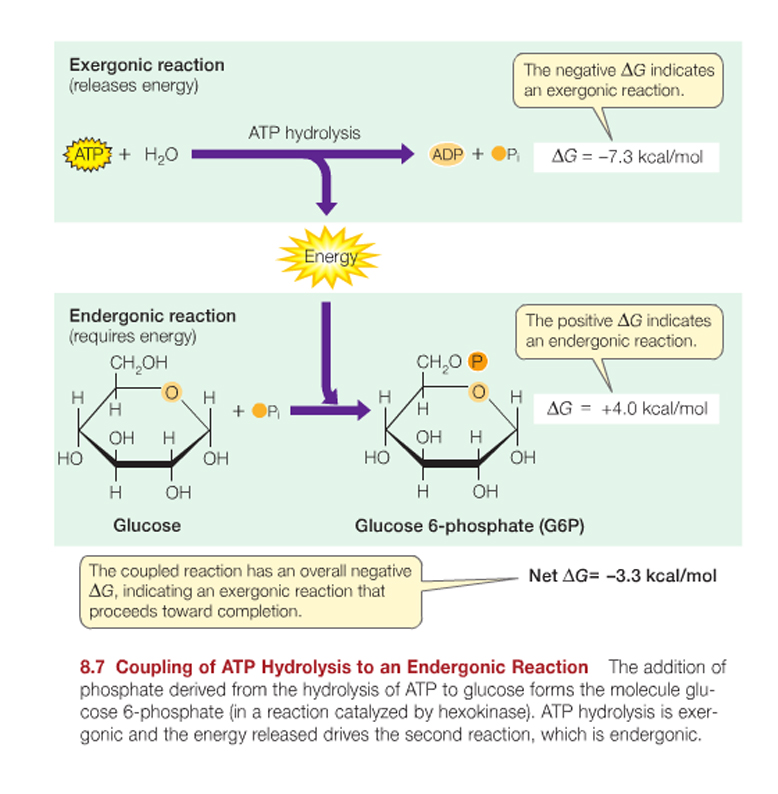
ATP hydrolysis releases energy -making it available for the cell to use elsewhere.....
ATP consists of the nitrogenous base adenine bonded to ribose. Carbon 5 of the ribose has three phosphate groups attached.
ATP can be hydrolyzed to yield ADP and an inorganic phosphate ion (Pi, short for HPO42-).
ATP + H2O ----> ADP + Pi + free energy.
The change in free energy (DG) ~ 7.3 kcal/mol at a living cell’s typical temperature and pH.
The equilibrium for this reaction is far to the right of the equation shown above, favouring the production of ADP ; so much so that there are approximately 1 x 107 ADP molecules to each remaining ATP.
Making ATP from ADP involves overcoming repulsive negative charges on the phosphates to be joined.
The energy that is necessary to do this in humans and many other living cells is generally stored as glucose (or condensed derivatives thereof....) or other fuel molecules and is released in the ensuing exergonic process.
<
Fats, a variety of sugars, and proteins can also be consumed for energy. However, a great many organisms (not all) preferentially use glucose as their primary fuel molecule.
As a consequence, almost all foods that are eventually used for energy are converted to glucose or to an intermediate metabolic product of its breakdown.
When burned in a flame, glucose releases heat, carbon dioxide, and water.
C6H12O6 + 6 O2 ---> 6 CO2 + 6 H2O + energy (heat and light).
The same equation applies for the biological, metabolic use of glucose. This process, however, has many steps and is carefully controlled.
When glucose is used up by the cell -as might be expected- about half of the energy is collected in ATP.
The delta G for the complete conversion of glucose is approximately - 686 kcal/mol.
The overall reaction is, therefore, highly exergonic, and it can consequently be used to drive the endergonic formation of a number of ATP's.
 and
and
Some types of cells are only able to metabolize glucose incompletely, while others do a much more efficient job and metabolize it completely.

About Quick Facts Academics |
Admissions Undergraduate Research |
Libraries University Library Campus Life
Housing |
Athletics Alumni
|
 |